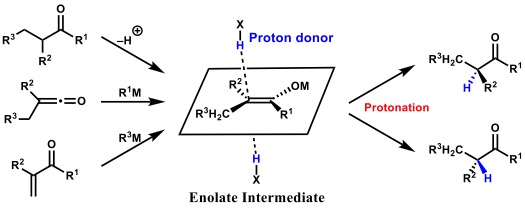
水中でのプロトン移動の高度制御
Proton transfer in water is the most fundamental phenomenon in redox processes and therefore it plays an essential role in biological processes such as muscle contraction, the electron transport chain in the mitochondrial matrix and enzyme catalysis. The enantioselective introduction of a proton, the smallest element in the periodic table, presents its own unique challenges, because it leads to non-selective quenching in proton-rich environments. The electron withdrawing group at α-position, which is required to stabilize the carbanion intermediate, would play an important role in increasing the stereocenter’s susceptibility to racemization. In addition, enolate intermediates can adopt E- or Z-geometries, generally leading to opposite stereoisomers upon protonation.
Topics
不斉プロトン化反応(チオール付加型 )
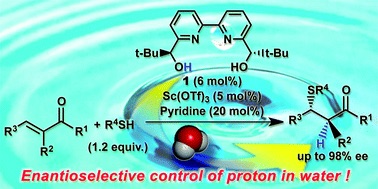
解説
Water was found to be the optimal solvent for tandem thia-Michael addition-protonation process, switching to organic solvents (CH2Cl2, THF, toluene, ethanol, and aqueous co-solvent system) resulted in diminished reactivity and poor stereoinduction. Despite a rapid racemic protonation cause by pyridine, the reaction proceeded significantly slower and all enantioselectivity was lost in the absence of pyridine. The ,-dimethyl substituted tetralones were effective to suppress the undesired base-catalyzed racemic pathway, displaying a significant boost in enantioselectivity.
論文へのアクセス
- Chiral-Sc catalyzed asymmetric Michael addition/protonation of thiols with enones in water
- T. Kitanosono, M. Sakai, M. Ueno, S. Kobayashi
- Org. Biomol. Chem. 7134-7147 (2012). DOI: 10.1039/C2OB26264A