水中直接型不斉アルドール反応への挑戦
Enzymes exhibit overwhelmingly superior catalysis compared with artificial catalysts. Current strategies to rival enzymatic catalysis require unmodified or minimally-modified structures of active sites, gigantic molecular weight, and sometimes the use of harsh conditions such as extremely low temperatures in organic solvents. Chemists have dreamed of rational design of enzyme-like catalyst and outdoing the natural wisdom. On the other hand, as mentioned in ”Highly selective Mukaiyama aldol reactions in aqueous environments” , the aldol reaction presents numerous challenges to chemists, including its reversibility, issues of chemo- and stereoselectivity, harshness of reaction conditions, and inhibition of side-reaction, which has spurred the development of many powerful stoichiometric processes to address these issues. Development of catalytic methods that avoid the production of stoichiometric by-products while maintaining the high levels of stereochemical control would provide an atom-economical alternative. Indeed, numerous catalytic methods have been reported in recent years, including enzymes, catalytic antibodies and organocatalyst-based small molecules. There are still many difficulties on catalyst loading, reactivity and selectivity. We sustain our efforts to realize a chemistry-based design of small molecules toward the simplest metalloenzyme-like catalysts that can function in water.
Reviews:
- Mukaiyama Aldol Reactions in Aqueous Media,
- T. Kitanosono, S. Kobayashi,
- Adv. Synth. Catal., 355, 3095-3118 (2013). DOI: 10.1002/adsc.201300798
- Development of Chiral Catalysts for Mukaiyama Aldol Reactions in Aqueous Media,
- T. Kitanosono, S. Kobayashi,
- Chem. Rec., 14, 130-143 (2014). DOI: 10.1002/tcr.201300040
Topics
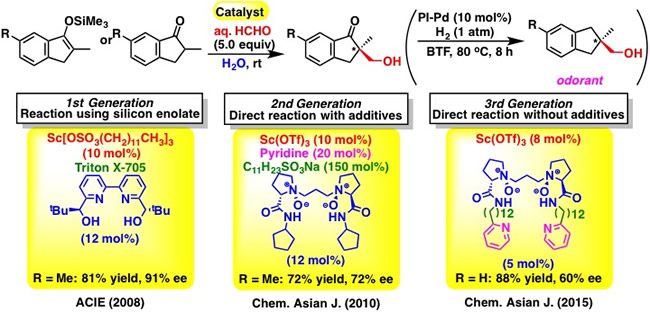
アルドラーゼを模倣した最も単純な人工金属酵素の開発 (第一世代)
Comment
First generation catalyst combined with surfactant for Mukaiyama aldol reaction of silyl enol ethers in water (2008)
A chiral scandium catalyst with N,N’-dioxide ligand was developed for direct-type aldol reaction using aqueous formaldehyde. The environment formed by a chiral scandium complex merged with surfactant (Lewis acid-surfactant–combined catalyst, abbreviated as LASC) was found to be efficient for the reaction of hydrophilic formaldehyde with hydrophobic silicon enolates in an enantioselective manner. After the completion of the reaction, the reaction mixture was centrifuged (3,000 rpm, 20 min) to separate the colloidal white dispersion into three phases that consist of aqueous, surfactant catalyst and organic phases, respectively.
Access to paper
- Lewis Acid Catalysis in Water with a Hydrophilic Substrate: Scandium-Catalyzed Hydroxymethylation with Aqueous Formaldehyde in Water
- M. Kokubo, C. Ogawa, S. Kobayashi
- Angew. Chem. Int. Ed. , 47, 6909-6911 (2008). DOI: 10.1002/anie.200801849
アルドラーゼを模倣した最も単純な人工金属酵素の開発 (第二世代)
Comment
Second generation catalyst combined with surfactant for direct-type aldol reaction of ketones in water (2010)
A chiral scandium catalyst with N,N’-dioxide ligand was developed for direct-type aldol reaction using aqueous formaldehyde. The environment formed by a chiral scandium complex merged with surfactant (Lewis acid-surfactant–combined catalyst, abbreviated as LASC) was found to be efficient for the reaction of hydrophilic formaldehyde with hydrophobic silicon enolates in an enantioselective manner. After the completion of the reaction, the reaction mixture was centrifuged (3,000 rpm, 20 min) to separate the colloidal white dispersion into three phases that consist of aqueous, surfactant catalyst and organic phases, respectively.
Access to paper
- Chiral Scandium-Catalyzed Enantioselective Hydroxymethylation of Ketones in Water
- S. Kobayashi, M. Kokubo, K. Kawasumi, T. Nagano
- Chem. Asian J. , 5, 490-492 (2010). DOI: 10.1002/asia.200900442
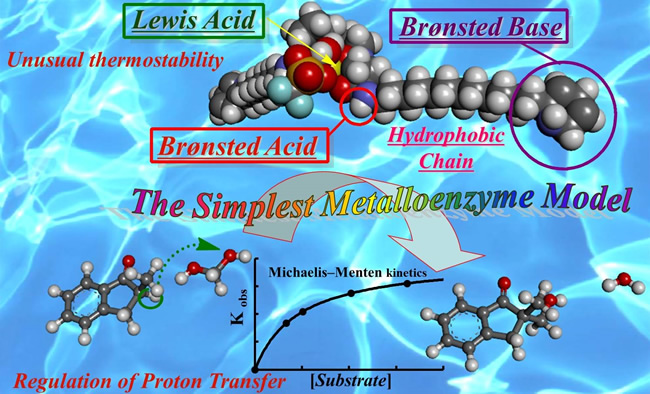
アルドラーゼを模倣した最も単純な人工金属酵素の開発 (第三世代)
Comment
Third generation function-integrated catalyst modeled after enzymatic system (2015)
A chiral scandium catalyst with N,N’-dioxide ligand was developed for direct-type aldol reaction using aqueous formaldehyde. The environment formed by a chiral scandium complex merged with surfactant (Lewis acid-surfactant–combined catalyst, abbreviated as LASC) was found to be efficient for the reaction of hydrophilic formaldehyde with hydrophobic silicon enolates in an enantioselective manner. After the completion of the reaction, the reaction mixture was centrifuged (3,000 rpm, 20 min) to separate the colloidal white dispersion into three phases that consist of aqueous, surfactant catalyst and organic phases, respectively.
Access to paper
- Toward Chemistry-Based Design of the Simplest Metalloenzyme-Like Catalyst That Works Efficiently in Water
- T. Kitanosono, S. Kobayashi
- Chem. Asian J. , 10, 133-138 (2015). DOI: 10.1002/asia.201403004