論文リスト
- 学術論文:2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002
- 総説・著書
学術論文 2016
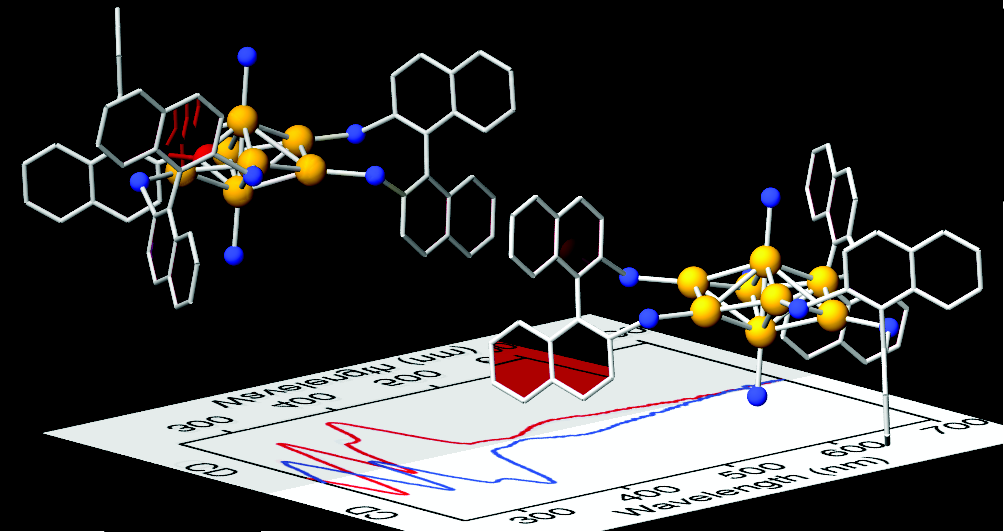
Amplification of Optical Activity of Gold Clusters by the Proximity of BINAP
Shinjiro Takano and Tatsuya Tsukuda*
J. Phys. Chem. Lett. 7, 4509-4513 (2016).![]()
Selected as ACS Editors' Choice
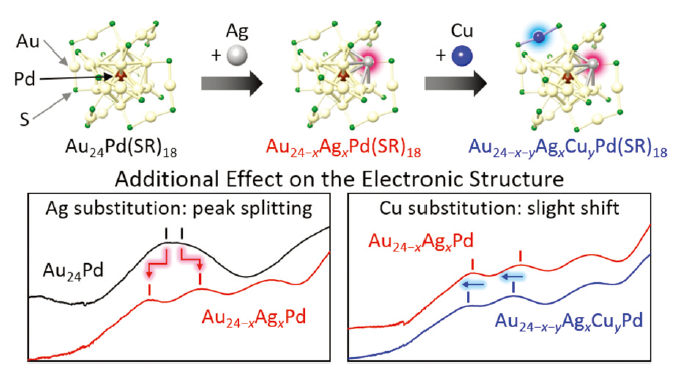
Tuning the electronic structure of thiolate-protected 25-atom clusters by co-substitution with metals having different preferential site
Sachil Sharma, Seiji Yamazoe, Tasuku Ono, Wataru, Kurashige, Yoshiki Niihori, Katsuyuki Nobusada,* Tatsuya Tsukuda,* and Yuichi Negishi*
Dalton Trans. 45, 18064-18068 (2016).![]()
Selected as Back Cover
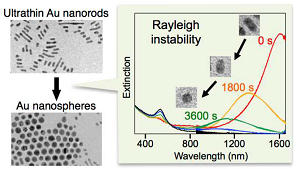
Rayleigh Instability and Surfactant-Mediated Stabilization of Ultrathin Gold Nanorods
Ryo Takahata, Seiji Yamazoe, Chompunuch Warakulwit, Jumras Limtrakul, and Tatsuya Tsukuda*
J. Phys. Chem. C 120, 17006-17010 (2016).![]()
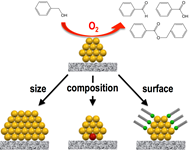
Controlled Synthesis of Carbon Supported Gold Clusters for Rational Catalyst Design
Seiji Yamazoe, Tatchamapan Yoskamtorn, Shinjiro Takano, Sudarat Yadnum, Jumras Limtrakul, and Tatsuya Tsukuda*
Chem. Rec. 16, 2338-2348 (2016).![]()
Invited Personal Account
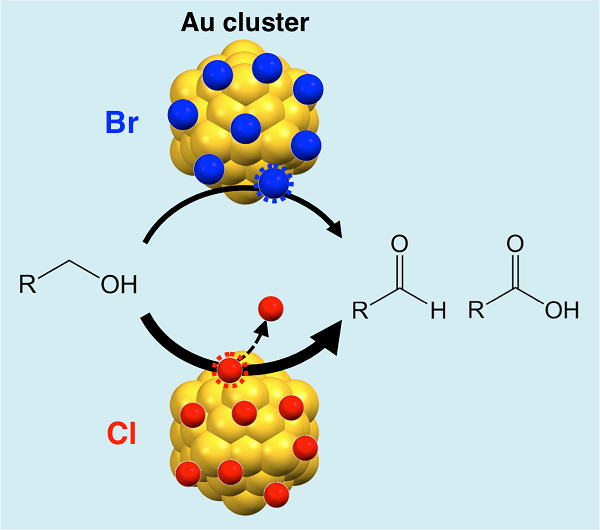
Halogen adsorbates on polymer-stabilized gold clusters: Mass spectrometric detection and effects on catalysis
Ryo Ishida, Setsuka Arii, Wataru Kurashige, Seiji Yamazoe, Kiichirou koyasu, Yuichi Negishi, and Tatsuya Tsukuda*
Chin. J. Catal. 37, 1656-1661 (2016).![]()
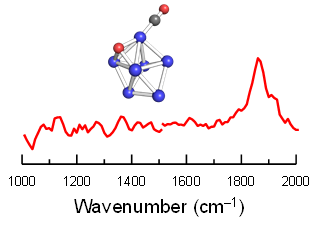
Size-Specific, Dissociative Activation of Carbon Dioxide by Cobalt Cluster Anions
Akimaro Yanagimachi, Kiichirou Koyasu, David Yubero Valdivielso, Sandy Gewinner, Wieland Schöllkopf, André Fielicke, and Tatsuya Tsukuda*
J. Phys. Chem. C 120, 14209-14215 (2016).![]()
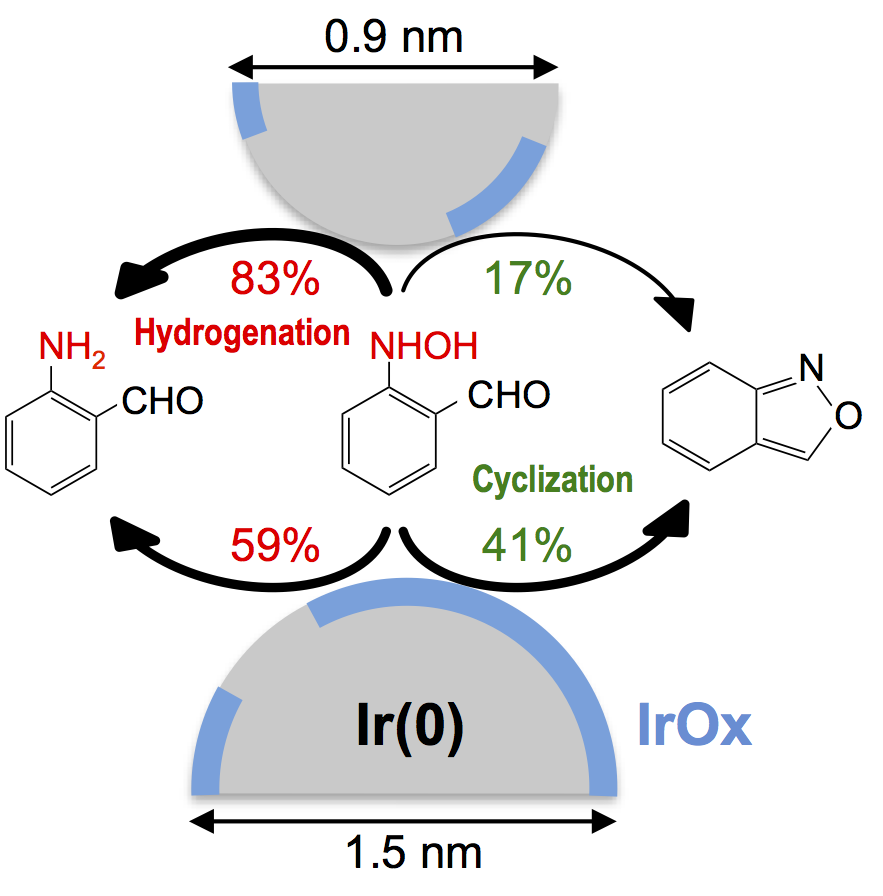
Partially Oxidized Iridium Clusters Within Dendrimers: Size-Controlled Synthesis and Selective Hydrogenation of 2-Nitrobenzaldehyde
Tatsuya Higaki, Hirokazu Kitazawa, Seiji Yamazoe, and Tatsuya Tsukuda*
Nanoscale 8, 11371-11374 (2016).![]()
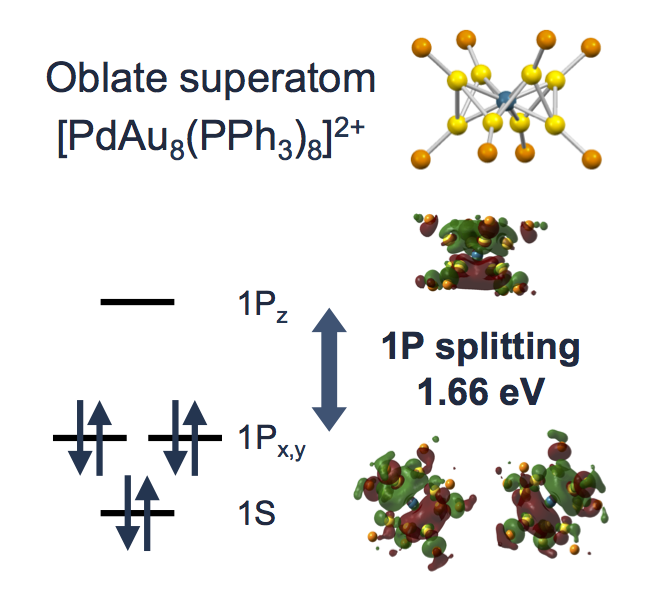
Selective and High-Yield Synthesis of Oblate Superatom [PdAu8(PPh3)8]2+
Shota Matsuo, Shinjiro Takano, Seiji Yamazoe, Kiichirou Koyasu, and Tatsuya Tsukuda*
ChemElectroChem 3, 1206-1211 (2016).![]()
Selected as Front Cover (Cover Profile)
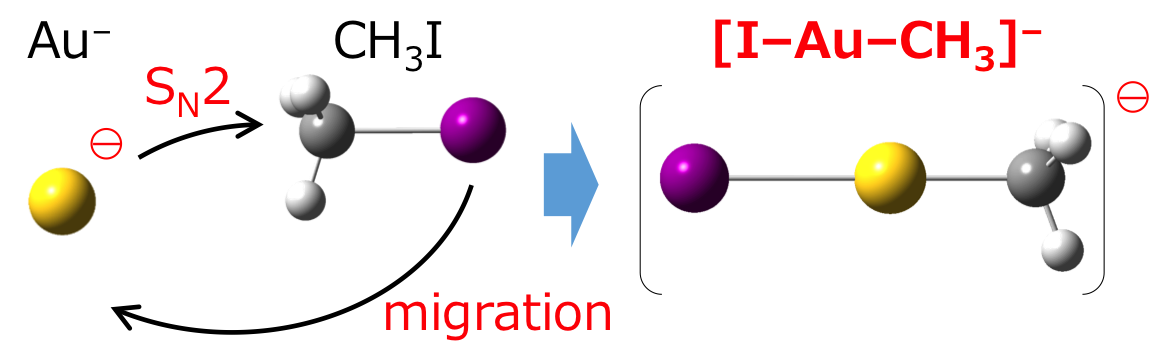
Oxidative Addition of CH3I to Au− in the Gas Phase
Satoru Muramatsu, Kiichirou Koyasu, and Tatsuya Tsukuda*
J. Phys. Chem. A 120, 957-963 (2016).![]()
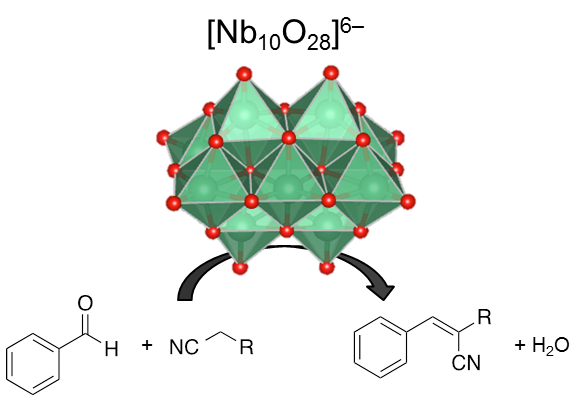
Application of Group V Polyoxometalate as Efficient Base Catalyst: a Case Study of Decaniobate Cluster
Shun Hayashi, Seiji Yamazoe, Kiichirou Koyasu, and Tatsuya Tsukuda*
RSC Advances 6, 16239-16242 (2016).![]()
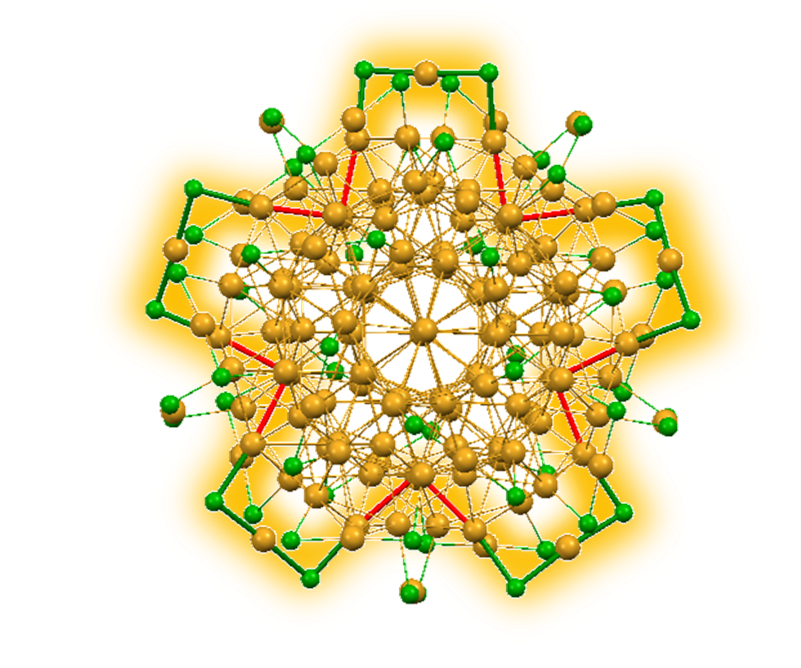
Hierarchy of Bond Stiffnesses within Icosahedral-based Gold Clusters Protected by Thiolates
Seiji Yamazoe, Shinjiro Takano, Wataru Kurashige, Toshihiko Yokoyama, Kiyofumi Nitta, Yuichi Negishi, and Tatsuya Tsukuda*
Nature Communications 7, 10414 (2016).![]()
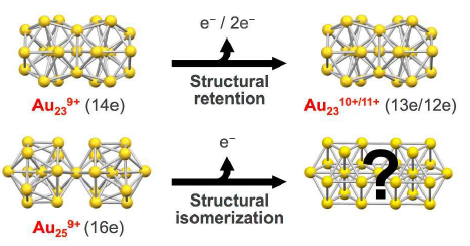
The Electrooxidation-induced Structural Changes of Gold Di-superatomic Molecules: Au23 vs. Au25
Shota Matsuo, Seiji Yamazoe, Jing-Qiang Goh, Jaakko Akola, and Tatsuya Tsukuda*
Phys. Chem. Chem. Phys. 18, 4822-4827 (2016).![]()
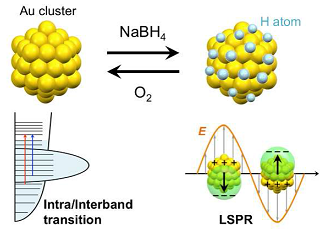
Repeated Appearance and Disappearance of Localized Surface Plasmon Resonance in 1.2 nm Gold Clusters Induced by Adsorption and Desorption of Hydrogen Atoms
Ryo Ishida, Seiji Yamazoe, Kiichirou Koyasu, and Tatsuya Tsukuda*
Nanoscale 8, 2544-2547 (2016).![]()
2015 Hot Papers in Nanoscale
Selected as inside front cover