論文リスト
- 学術論文:2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002
- 総説・著書
学術論文 2005
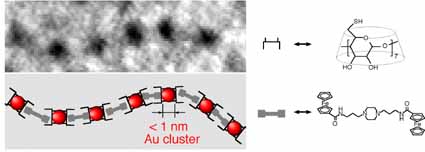
Subnanometer-Sized Gold Clusters with Dual Molecular Receptors: Synthesis and Assembly in One-Dimensional Arrangements
Y. Negishi, H. Tsunoyama, Y. Yanagimoto, and T.
Tsukuda*
Chem.
Lett., 34, 1638-1639 (2005). ![]()
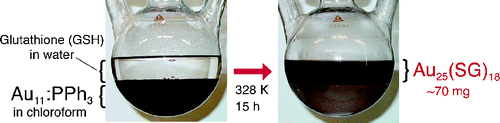
Large-Scale Synthesis of Thiolated Au25 Clusters via Ligand Exchange Reactions of Phosphine-Stabilized Au11 Clusters
Y. Shichibu, Y. Negishi, T. Tsukuda*, and T.
Teranishi*
J.
Am. Chem. Soc. (communications), 127,
13464-13465 (2005). ![]()
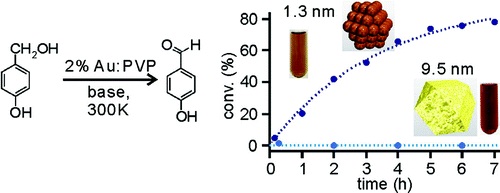
Size-Specific Catalytic Activity of Polymer-Stabilized Gold Nanoclusters for Aerobic Alcohol Oxidation in Water
H. Tsunoyama, H. Sakurai, Y. Negishi, and T. Tsukuda*
J.
Am. Chem. Soc. (communications), 127, 9374-9375
(2005). ![]()
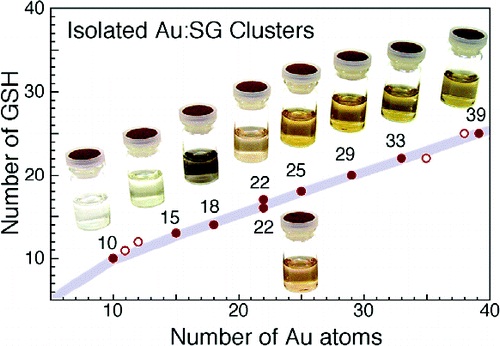
Glutathione-Protected Gold Clusters Revisited: Bridging the Gap between Gold(I)-Thiolate Complexes and Thiolate-Protected Gold Nanocrystals
Y. Negishi, K. Nobusada, and T. Tsukuda*
J.
Am. Chem. Soc. (communications), 127, 5261-5270
(2005). ![]()