論文リスト
- 学術論文:2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002
- 総説・著書
学術論文 2013
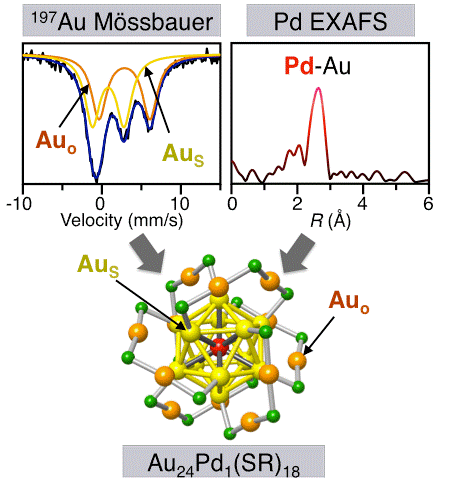
Formation of a Pd@Au12 Superatomic Core in Au24Pd1(SC12H25)18 Probed by 197Au Mössbauer and Pd K-edge EXAFS Spectroscopy
Y. Negishi, W. Kurashige, Y. Kobayashi, S. Yamazoe,
N. Kojima, M. Seto, T. Tsukuda*
J. Phys. Chem. Lett., 4,
3579-3584 (2013). ![]()
Selenolate-protected Au38 Nanoclusters: Isolation and
Structural Characterization
W. Kurashige, S. Yamazoe, K. Kanehira, T. Tsukuda,
Y. Negishi
J. Phys. Chem. Lett., 4, 3181-3185 (2013). ![]()
Direct Atomic Imaging and Density Functional Theory Study of Au24Pd1 Cluster Catalyst
A. Bruma, F. R. Negreiros, S. Xie, T. Tsukuda, R. L. Johnston, A. Fortunelli, Z. Y. Li
Nanoscale, 5,
9620-9625 (2013). Selected as front cover![]()

Binding Motif of Terminal Alkynes on Gold Clusters
P. Maity, S. Takano, S. Yamazoe, T. Wakabayashi, T.
Tsukuda*
J. Am. Chem. Soc. 135, 9450-9457 (2013). ![]()
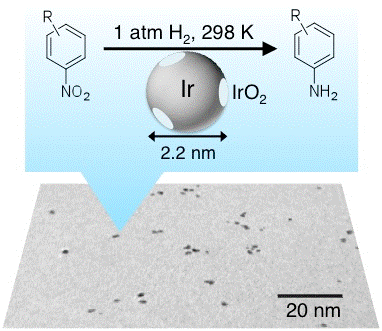
Selective Hydrogenation of Nitroaromatics by Colloidal Iridium Nanoparticles
Md. J. Sharif, P. Maity, S. Yamazoe, T. Tsukuda*
Chem. Lett., 42, 1023-1025 (2013). Editor's choice ![]()
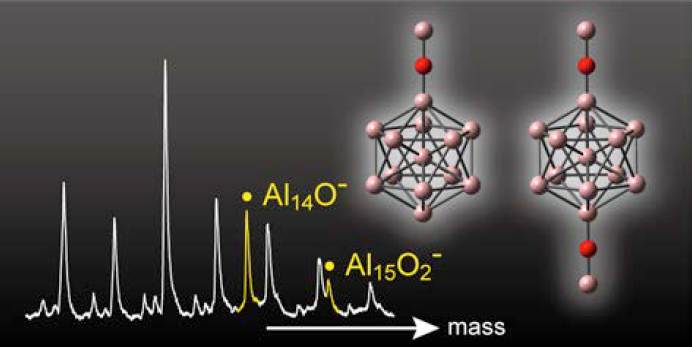
Structural Characterization of Unprecedented Al14O– and Al15O2–: Photoelectron Spectroscopy and Density Functional Calculations
T. Watanabe, T. Tsukuda*
J. Phys. Chem. C, 117, 6664-6668 (2013). ![]()
Correction to "Dendrimer Encapsulated Copper Cluster as a Chemoselective and Regenerable Hydrogenation Catalyst"
P. Maity, S. Yamazoe, T. Tsukuda*
ACS
Catal., 3, 554 (2013).
![]()
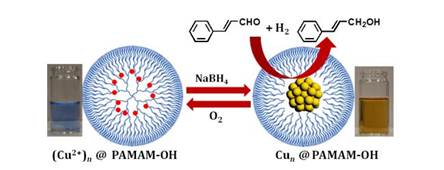
Dendrimer Encapsulated Copper Cluster as a Chemoselective and Regenerable Hydrogenation Catalyst
P. Maity, S. Yamazoe, T. Tsukuda*
ACS Catal.,
3, 182-185 (2013). ![]()
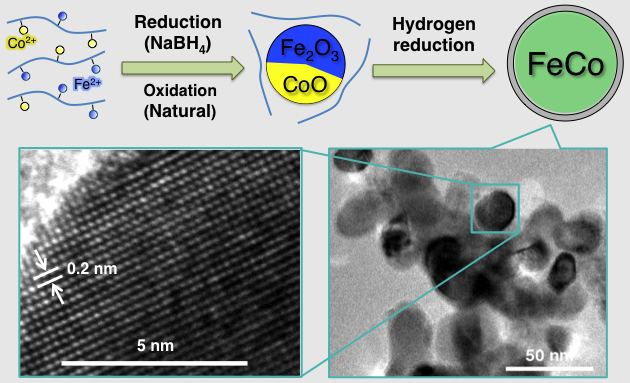
Enhanced Magnetization in Highly-Crystalline and Atomically-Mixed bcc Fe-Co Nanoalloys Prepared by Hydrogen Reduction of Oxide Composites
Md Jafar Sharif, M. Yamauchi,* S. Toh, S.
Matsumura, S. Noro, K. Kato, M. Takata, T. Tsukuda
Nanoscale, 5, 1489-1493 (2013). ![]()
Structural Evolution of Glutathionate-protected Gold Clusters Studied by Means of 197Au Mössbauer Spectroscopy
N. Kojima,* Y. Kobayashi, Y. Negishi, M. Seto, T.
Tsukuda
Hyperfine Interactions, 217, 91-98 (2013).
![]()
Production of Oxidation-Resistant Copper Nanoparticles on Carbon Nanotubes by Photoreduction
N. Nishida, A. Miyashita, T. Tsukuda, H. Tanaka*
Chem. Lett., 42, 168-170 (2013).
![]()
