_¶Xg
- wp_¶F2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002
- àE
wp_¶ 2012
Size Control of Ni Nanocluster by the Carbon Chain Length of Secondary Alkoxide
H. Kitagawa, N. Ichikuni*, T. Hara, S. Shimizu, S.
Xie, T. Tsukuda
e-J.
Surf. Sci. Nanotech., 10,
648-650 (2012). ![]()
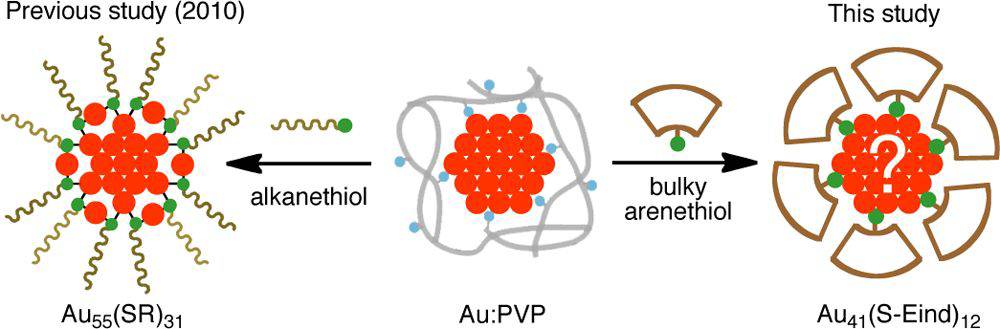
A New Binding Motif of Sterically Demanding Thiolates on a Gold Cluster
Jun-ichi Nishigaki, Risako Tsunoyama, Hironori
Tsunoyama, Nobuyuki Ichikuni, Seiji Yamazoe, Yuichi Negishi, Mikinao
Ito, Tsukasa Matsuo, Kohei Tamao, and Tatsuya Tsukuda*
J.
Am. Chem. Soc., 134, 14295-14297 (2012). ![]()
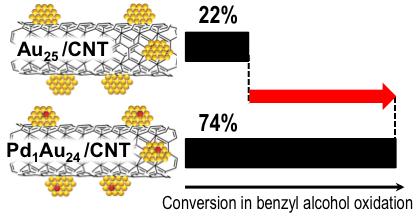
Enhancement in Aerobic Alcohol Oxidation Catalysis of Au25 Clusters by Single Pd Atom Doping
S. Xie, H. Tsunoyama, W. Kurashige, Y. Negishi, T.
Tsukuda*
ACS
Catal., 2, 1519-1523 (2012) ![]()
Synthesis and the Origin of the Stability of Thiolate-Protected Au130 and Au187 Clusters
Y. Negishi*, C. Sakamoto, T. Ohyama, T. Tsukuda
J.
Phys. Chem. Lett., 3, 1624-1628 (2012) ![]()
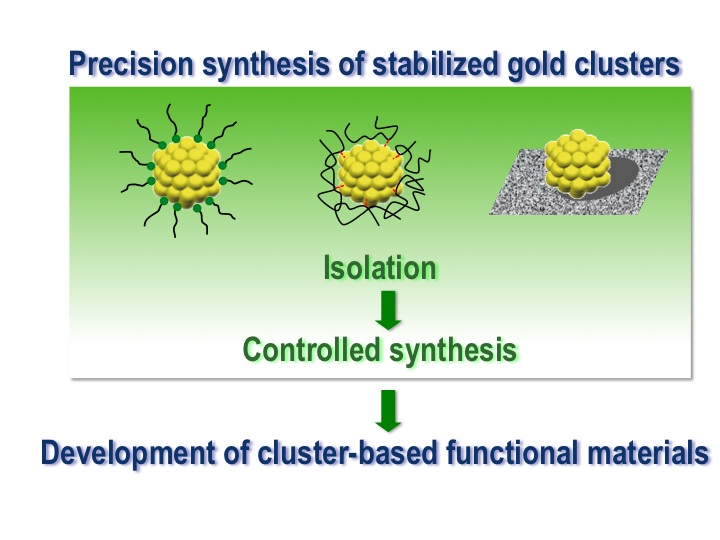
Stabilized Gold Clusters: from Isolation Toward Controlled Synthesis
Prasenjit Maity, Songhai Xie, Miho Yamauchi and
Tatsuya Tsukuda*
Nanoscale, 4, 4027-4037 (2012) ![]()
Invited Review, Highly-cited Paper (Thomson Reuters)
Preparation and Catalysis of Supported NiO Nanocluster for Oxidative Coupling of Thiophenol
Nobuyuki Ichikuni*, Osamu Tsuchida, Jun Naganuma,
Takayoshi Hara, Hironori
Tsunoyama, Tatsuya Tsukuda and Shogo Shimazu
Trans. MRS-J , 37, 177-180 (2012).![]()
Size and Shape of Nanoclusters: Single-shot Imaging Approach
Y. Han, D.S. He, Y. Liu. S. Xie, T. Tsukuda, Ziyou
Li*
Small 8, 2361-2364 (2012)
![]()
Selective Synthesis of Organogold Magic Clusters Au54(CßCPh)26
Prasenjit Maity, Tomonari Wakabayashi, Nobuyuki
Ichikuni, Hironori Tsunoyama, Songhai Xie, Miho Yamauchi, and Tatsuya
Tsukuda*
Chem. Commun. 48,
6085-6087 (2012) ![]() Selected as front cover of Chem. Commun.
Selected as front cover of Chem. Commun.

Platonic Hexahedron Composed of Six Organic Faces with an Inscribed Au Cluster
Masanori Sakamoto, Daisuke Tanaka, Hironori
Tsunoyama, Tatsuya Tsukuda, Yoshihiro Minagawa, Yutaka Majima, and
Toshiharu Teranishi*
J. Am. Chem. Soc., 134 (2),
816-819 (2012) ![]()
Study of the Structure and Electronic State of Thiolate-protected Gold Clusters by Means of 197Au Mössbauer Spectroscopy
N. Kojima*, K. Ikeda, Y. Kobayashi, T. Tsukuda, Y.
Negishi, G. Harada, T. Sugawara, M. Seto
Hyperfine
Interactions, 207 (1-3), 127-131(2012) ![]()
Thermal Stabillization of Thin Gold Nanowires by Surfactant-Coating: A Molecular Dynamic Study
S. E. Huber*, C. Warakulwit, J. Limtrakul, T.
Tsukuda, M. Probst
Nanoscale, 4, 585-590 (2012)
![]()
Toward an Atomic-Level Understanding of Size-Specific Properties of Protected and Stabilized Gold Clusters
Tatsuya Tsukuda*
Bull. Chem. Soc. Jpn., 85 (2), 151-168 (2012) ![]()
Invited Account, 2011-2013 First Half Hot Article, 2012-2014 First-quarter Hot Article, Highly-cited Paper (Thomson Reuters)
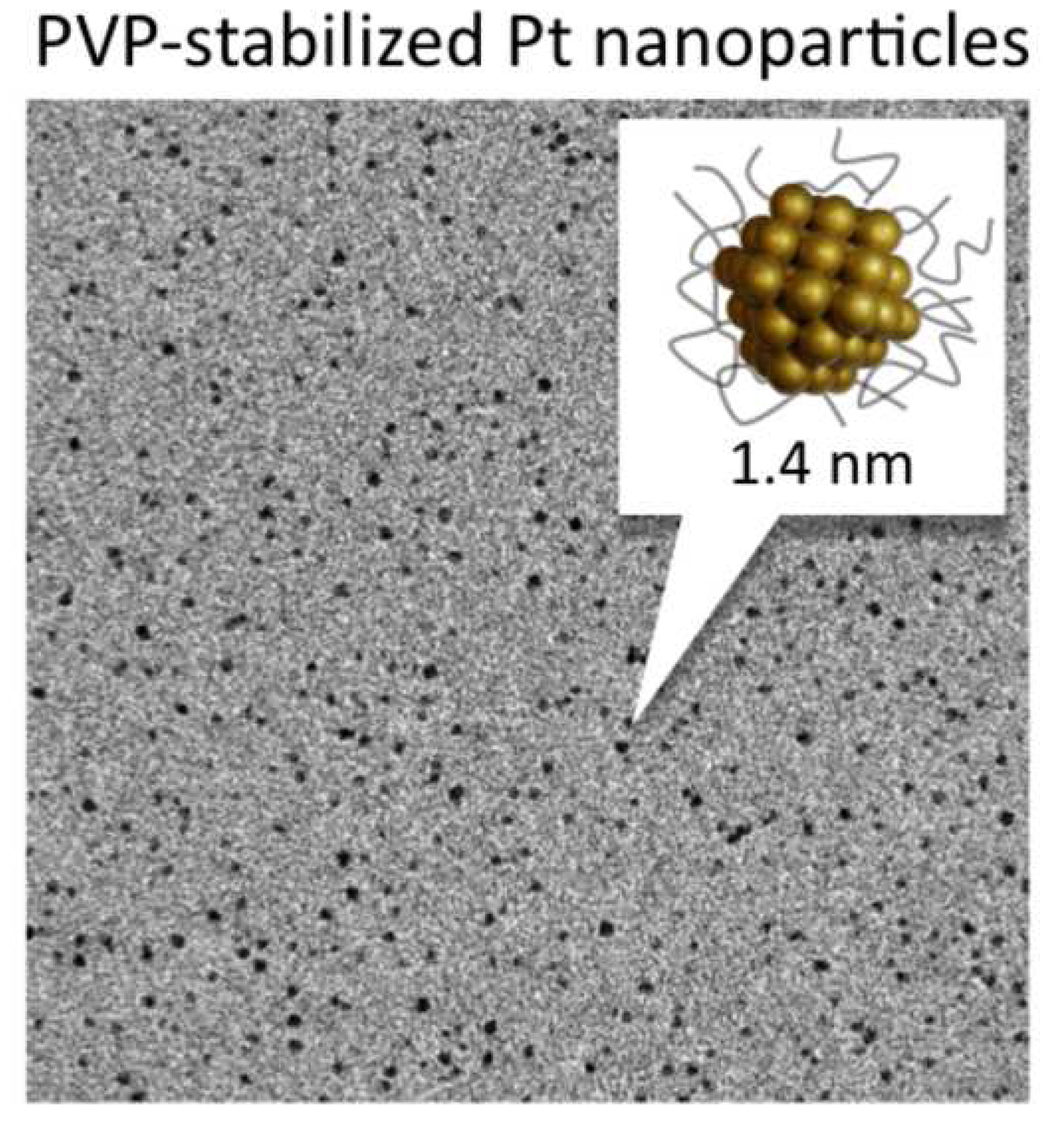
High-yield Synthesis of PVP-stabilized Small Pt Clusters by Microfluidic Method
M. Jakir Hossain, Hironori Tsunoyama, Miho Yamauchi, Nobuyuki Ichikuni, Tatsuya Tsukuda*
Catal. Today, 183, 101-107 (2012). ![]()
*Selected as featured article in Chemical Engineering of Advances in Enginerring