論文リスト
- 学術論文:2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002
- 総説・著書
学術論文 2011
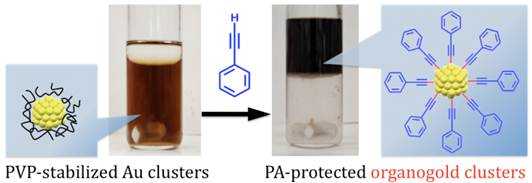
Organogold Clusters Protected by Phenylacetylene
P. Maity, H. Tsunoyama, M. Yamauchi, S. Xie, T.
Tsukuda*
J.
Am. Chem. Soc., 133 (50), 20123-20125 (2011)
![]()
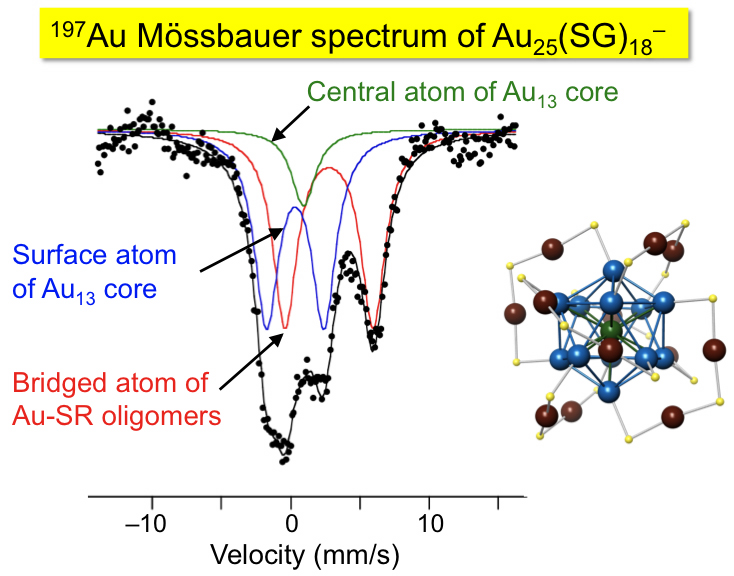
197Au Mössbauer Spectroscopy of Au25(SG)18- Revisited
Tatsuya Tsukuda*, Yuich Negishi, Yasushi Kobayashi,
Norimichi Kojima
Chemistry Letters, , 40 (11),
1292-1293 (2011). ![]()
Size-Controlled Synthesis of Gold Clusters as Efficient Catalysts for Aerobic Oxidation
Hironori Tsunoyama, Yongmei Liu, Tomoki Akita,
Nobuyuki Ichikuni, Hidehiro Sakurai, Songhai Xie. E, Tatsuya Tsukuda*
Catal.
Surv. Asia, 15, 230-239 (2011). ![]()
Invited
Isolation and Structural Characterization of Magic Silver Clusters Protected by 4-(Tert-butyl)benzyl Mercaptan
Yuichi Negishi*, Rio Arai, Yoshiki Niihori and
Tatsuya Tsukuda
Chem.
Commun., 47 (20), 5693-5695 (2011). ![]()
Synthesis and Characterization of Au102(p-MBA)44 Nanoparticles
Yael Levi-Kalisman, Pablo D. Jadzinsky, Nir
Kalisman, Hironori Tsunoyama, Tatsuya Tsukuda, David A. Bushnell, and
Roger D. Kornberg*
J.
Am. Chem. Soc., 133 (9), 2976-2982 (2011).
![]()
Production of an Ordered (B2) CuPd Nanoalloy by Low-temperature Annealing under Hydrogen Atmosphere
Miho Yamauchi*, Tatsuya Tsukuda
Dalton
Transaction, 40, 4842-4845 (2011). ![]()
Highly Selective Ammonia Synthesis from Nitrate with Photocatalytically Generated Hydrogen on CuPd/TiO2
Miho Yamauchi*, Ryu Abe, Tatsuya Tsukuda, Kenichi
Kato, and Masaki Takata
J.
Am. Chem. Soc., 133 (5), 1150-1152 (2011). ![]()
Aerobic Oxidations Catalyzed by Colloidal Nanogold
Tatsuya Tsukuda*, Hironori Tsunoyama, and Hidehiro
Sakurai
Chemistry-An Asian Journal, 6, 736-748 (2011). ![]()
Invited Focus Review
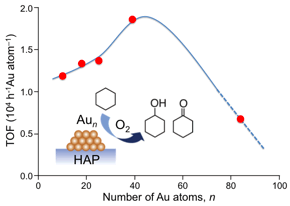
Aerobic Oxidation of Cyclohexane Catalyzed by Size-Controlled Au Clusters on Hydroxyapatite: Size Effect in the Sub-2 nm Regime
Yongmei Liu, Hironori Tsunoyama, Tomoki Akita,
Songhai Xie, and Tatsuya Tsukuda*
ACS
Catalysis, 1, 2-6 (2011). ![]()