論文リスト
- 学術論文:2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002
- 総説・著書
学術論文 2006
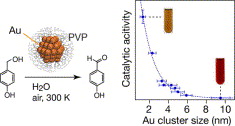
Size Effect on the Catalysis of Gold Clusters Dispersed in Water for Aerobic Oxidation of Alcohol
H. Tsunoyama, H. Sakurai, T. Tsukuda*
Chem.
Phys. Lett., 429, 528-532 (2006). ![]()
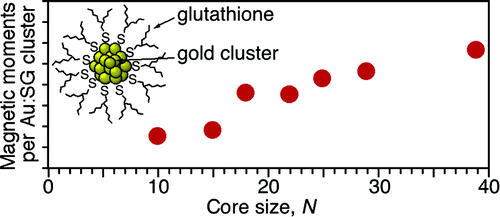
X-ray Magnetic Circular Dichroism of Size-Selected, Thiolated Gold Clusters
Y. Negishi, H. Tsunoyama, M. Suzuki, N. Kawamura,
M. M. Matsushita, K. Maruyama, T. Sugawara, T. Yokoyama*, and T.
Tsukuda*
J.
Am. Chem. Soc. (communications), 128,
12034-12035 (2006). ![]()
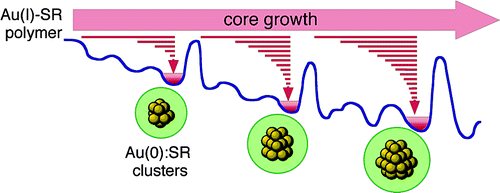
Kinetic Stabilization of Growing Gold Clusters by Passivation with Thiolates
Y. Negishi, Y. Takasugi, S. Sato, H. Yao, K.
Kimura, and T. Tsukuda*
J.
Phys, Chem. B (Letters), 110, 12218-12221 (2006).
![]()
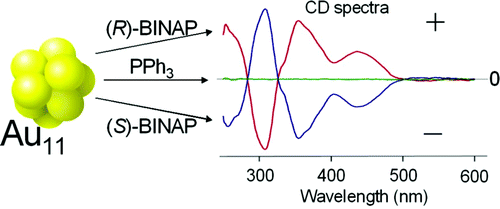
Chiroptical Activity of BINAP-stabilized Undecagold Clusters
Y. Yanagimoto, Y. Negishi, H. Fujihara, and T.
Tsukuda*
J.
Phys, Chem. B (Letters), 110, 11611-11614 (2006).
![]()
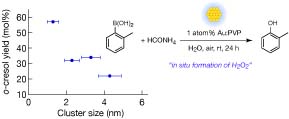
Aerobic Oxidation Catalyzed by Gold Nanocluster as a quasi-Homogeneous Catalyst: Application to Generation of Hydrogen Peroxide using Ammonium Formate
H. Sakurai,* H. Tsunoyama, and T. Tsukuda*
Trans. MRS-J., 31, 521-524 (2006).
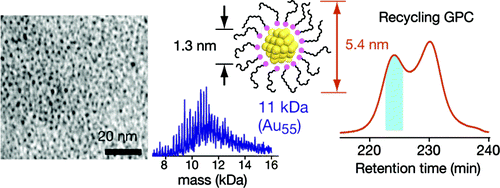
Chromatographic Isolation of "Missing" Au55 Clusters Protected by Alkanethiolates
H. Tsunoyama, Y. Negishi, and T. Tsukuda*
J.
Am. Chem. Soc. (communications), 128, 6036-6037
(2006). ![]()