Development of useful synthetic reactions using fluorenylidene as a protecting and activation group
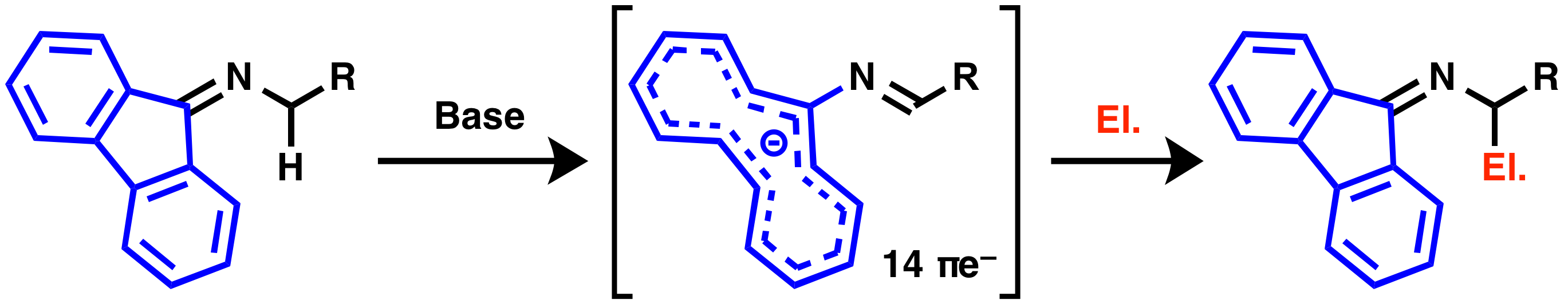
Fluorenylidene group has been known as a protecting group for primary amines, and it also can stabilize carbanion formed at the α-position of nitrogen atom strongly by its 14π electron conjugation system. Currently we are focusing on development of new synthetic methodologies by using fluorenylidene as a protecting and activation group.
Topics
- Catalytic Mannich-type reactions of α-aminoesters and α-aminophosphonates bearing fluorenylidene group
- Catalytic activation of simple aminoalkanes using fluorenylidene group
- Catalytic activation of α-aminoacetonitrile using fluorenylidene group
- Development of catalytic aldol-type reactions using fluorenylidene group
- Development of catalytic imine-imine cross-coupling reaction
Catalytic Mannich-type reactions of α-aminoesters and α-aminophosphonates bearing fluorenylidene group
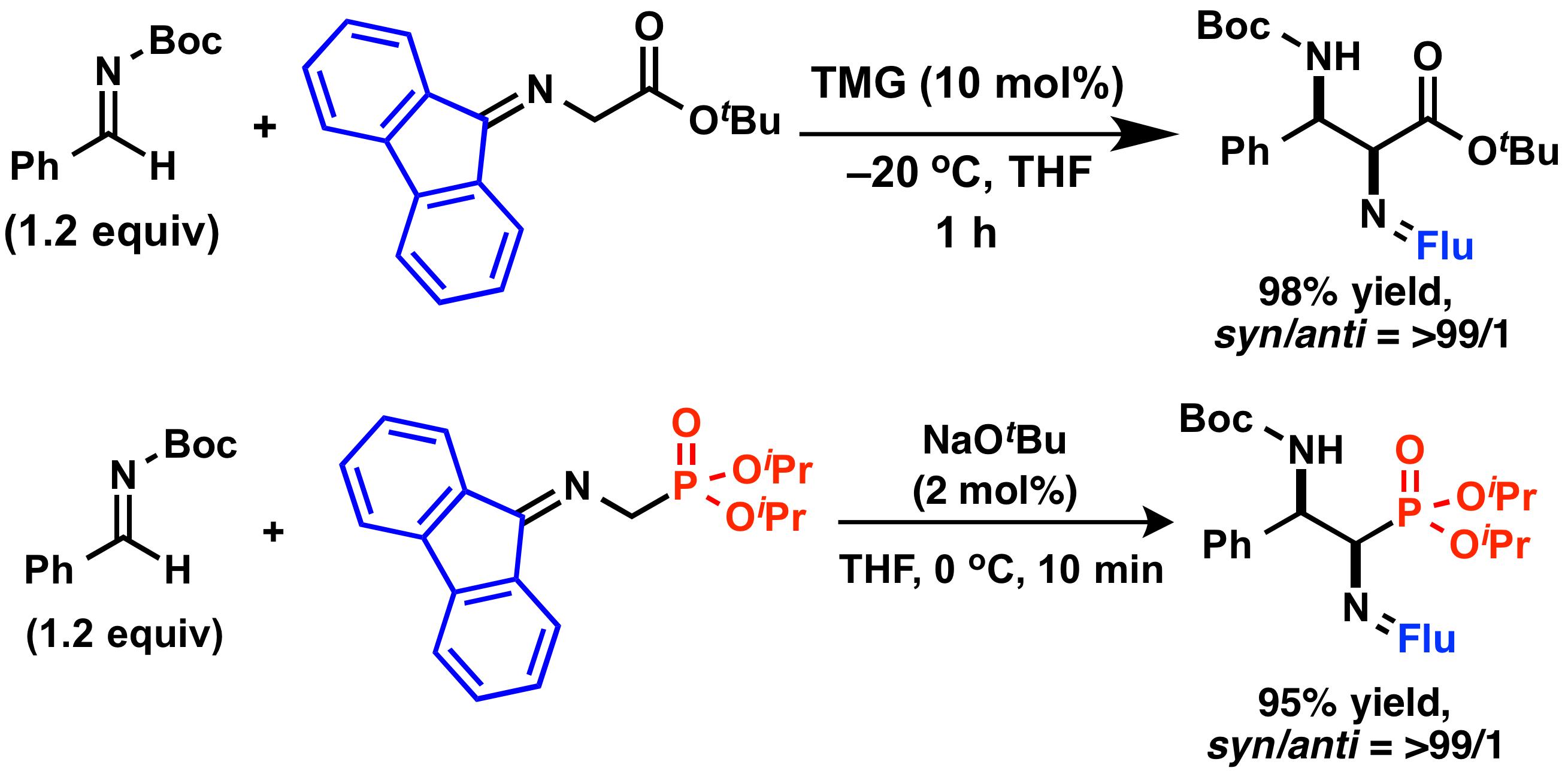
Comment
We revealed that fluorenylidene-protected α-aminoesters and α-aminophosphonates showed higher reactivity than benzophenone-protected ones in catalytic Mannich-type reactions. The desired reactions proceeded smoothly under mild reaction conditions in high yields with high diastereosectivities. Furthermore, their asymmetric variants also proceeded in high enantioselectivities by using a chiral organobase as a catalyst.
Access to paper
- The fluorenone imines of glycine esters and their phosphonic acid analogues
- Kobayashi, S.; Yazaki, R.; Seki, K.; Yamashita, Y.
- Angew. Chem. Int. Ed. 47, 5613 (2008). DOI: 10.1002/anie.200801322
Catalytic activation of simple aminoalkanes using fluorenylidene group
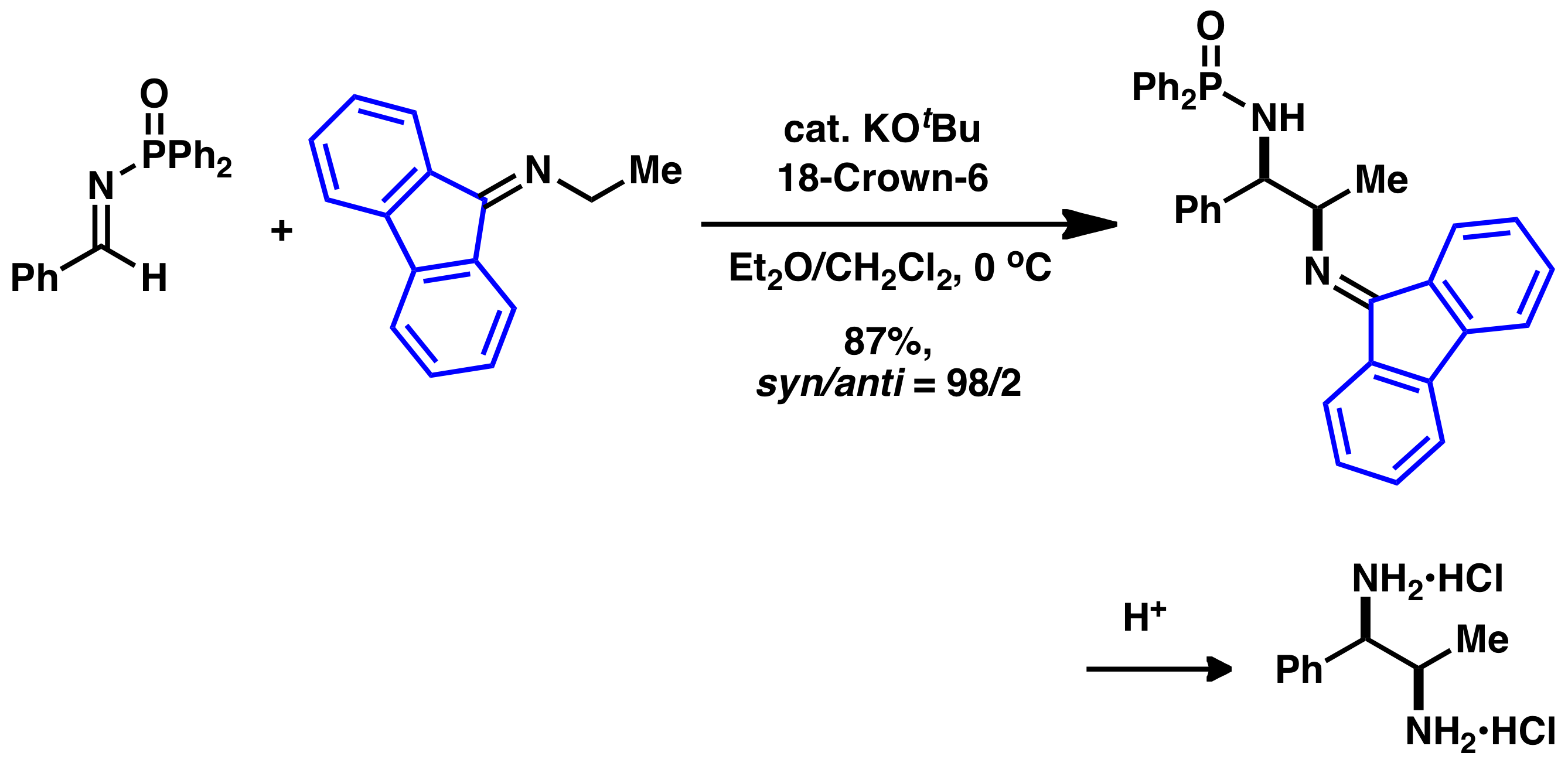
Comment
Aminoalkanes are potentially useful carbon nucleophiles, however, due to low acidity of the α-hydrogen, nitroalkanes have been employed as their synthetic equivalents. We have revealed that the fluorenylidene group also could activate α-hydrogens of simple amioalkanes, and catalytic Mannich-type reactions of fluorenylidene-protected aminoalkanes proceeded in high yields with high diastereoselectivities in the presence of a base catalyst. The products obtained were converted into 1,2-diamines by acid hydrolysis.
Access to paper
- Catalytic Carbon-Carbon Bond Forming Reactions of Aminoalkane Derivatives with Imines
- Chen, Y.-J.; Seki, K.; Yamashita, Y.; Kobayashi, S.
- J. Am. Chem. Soc. 132, 3244 (2010). DOI: 10.1021/ja909909q
Catalytic activation of α-aminoacetonitrile using fluorenylidene group

Comment
Cyano group is a useful functional group because it can be transformed into carboxylic acid derivatives and aminomethyl group. We have also developed catalytic Mannich-type reactions of fluorenylidene-protected α-aminoacetonitrile. Asymmetric variant of this reaction was also reported.
Access to paper
- Catalytic Mannich-Type Reactions of a-Aminoacetonitrile Using Fluorenylidene as a Protecting and Activating Group
- Yamashita, Y.; Matsumoto, M.; Chen, Y.-J.; Kobayashi, S.
- Tetrahedron 68, 7558 (2012). DOI: 10.1016/j.tet.2012.06.044
Catalytic aldol reaction using fluorenylidene group
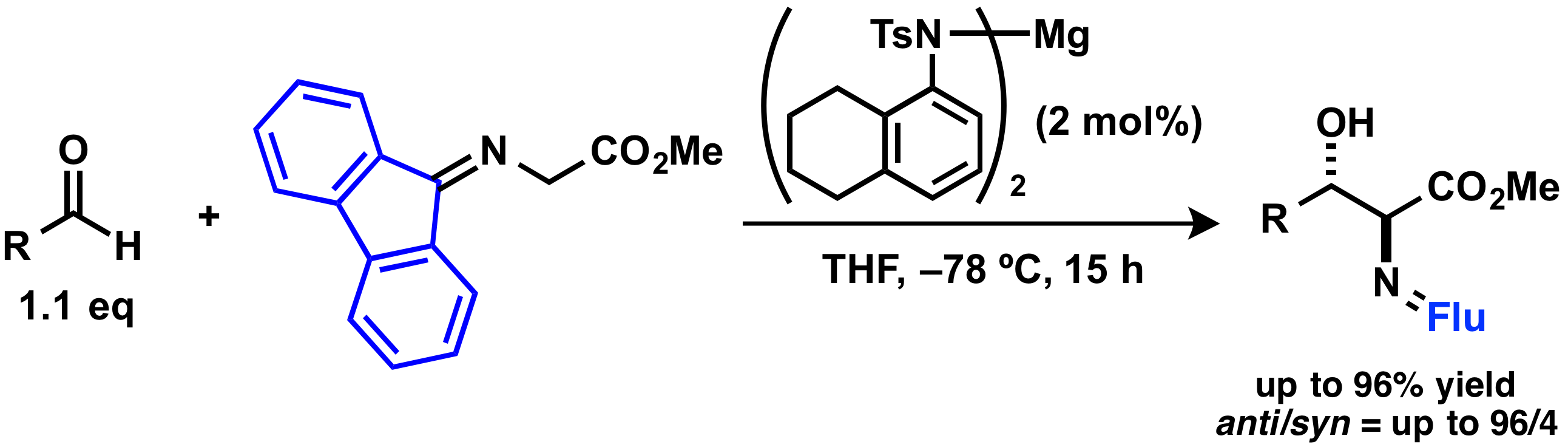
Comment
Catalytic direct-type aldol reaction of a glycine Schiff base is a useful reaction to provide β-hydroxy-α-aminoesters. We have revealed that catalytic aldol reactions of a fluorenylidene-protected α-aminoester proceeded smoothly even at low temperature with high diastereoselectivities in the presence of a magnesium salt as weak base. Reactivity of the fluorenylidene-protected α-aminoester was clearly higher than that of benzophenone-protected α-aminoester.
Access to paper
- Direct-Type Aldol Reactions of Fluorenylidene-Protected/Activated Glycine Esters with Aldehydes for the Synthesis of β-Hydroxy-α-Amino Acid Derivatives
- R. Rahmani, Matsumoto, M.; Yamashita, Y.; Kobayashi, S.
- Chem. Asian J. 7, 1191 (2012). DOI: 10.1002/asia.201200081
Catalytic Imine-Imine Cross-Coupling Reaction
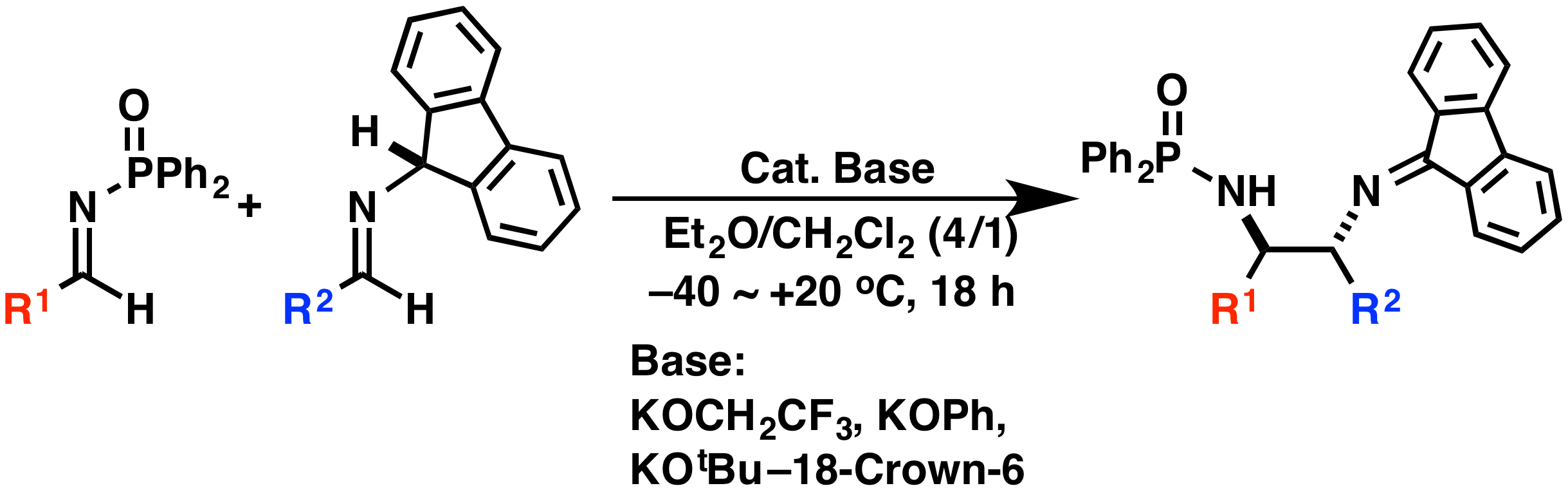
Comment
Imine-imine cross coupling reaction is one of the most efficient methods to prepare 1,2-diamine structures. In general, a stoichiometric amount of reductant is required for the reaction, and it is difficult to suppress homo-coupling reaction. Therefore, catalytic highly stereoselective imine-imine cross coupling reaction has been known to be very difficult. We have developed the first example of catalytic imine-imine cross coupling reactions by using fluorenyl group as a protecting group for nucleophilic imines. The desired reactions proceeded smoothly in the presence of a catalytic amount of base. The fluorenyl group was oxidized to fluorenylidene group during the coupling reaction.
Access to paper
- Catalytic Imine-Imine Cross-Coupling Reaction
- Matsumoto, M.; Harada, M.; Yamashita, Y.; Kobayashi, S.
- Chem. Commun. 50, 13041 (2014). DOI: 10.1039/C4CC06156J