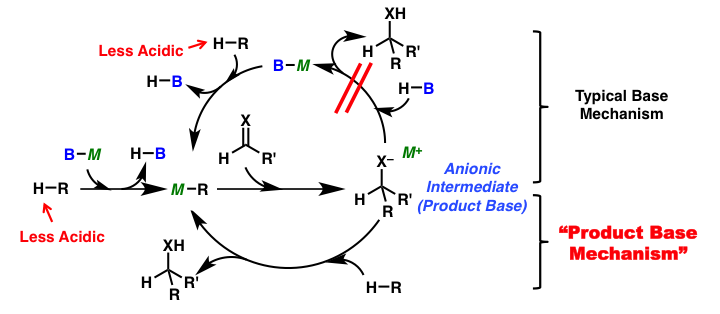
Development of methodologies for activation of less reactive substrates
Carbon-carbon bond forming reactions are important methods to construct complex molecular structures. Many types of the reactions have been developed and applied for the synthesis of complex molecules. Among them, reactions using only a catalytic amount of base are very efficient from a viewpoint of atom economy; however, one equivalent of a strong base is normally required to form the carbanion species when substrates with less acidic hydrogen atoms are used as pro-nucleophiles. Therefore, the development of new methodologies to activate these less reactive substrates and their application to catalytic reactions are desired. Recently, we have investigated catalytic reactions using a strong base via formation of a strongly basic intermediate, product base, which can deprotonate the next substrate directly without catalyst regeneration (product base mechanism), and found that catalytic carbon-carbon bond forming reactions of less acidic pro-nucleophiles, such as esters and amides bearing no EWG group at the α-position, proceeded smoothly.
Topics
- Development of catalytic Mannich-type reactions of simple esters.
- Development of catalytic asymmetric direct-type 1,4-addition reactions of simple amides and esters
Development of catalytic Mannich-type reactions of simple esters
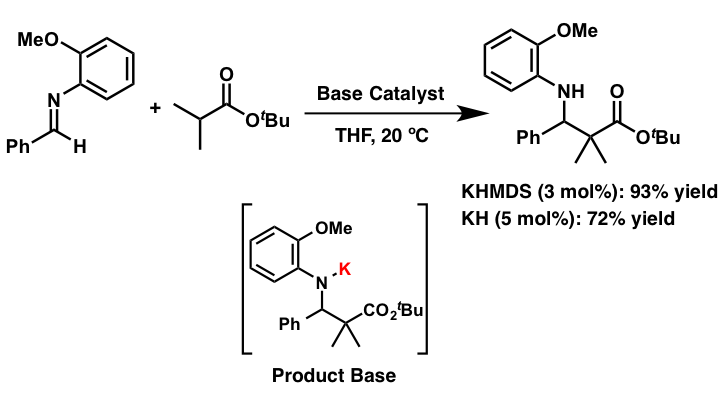
Comment
Ester and amides without EWG group at the α-position are less reactive to acid/base catalysis due to their less acidic nature when compared to ketones and aldehydes. Therefore a stoichiometric amount of a strong base is normally required to form reactive carbanion species for nucleophilic addition reactions. We developed catalytic direct Mannich-type reactions of simple esters, without EWG group at their α-position, via formation of well-designed strong basic intermediates by using a catalytic amount of potassium hydride (KH) as a strong Brønsted base.
Access to paper
- Development of Strong Brønsted Base Catalysis: Catalytic Direct-type Mannich Reactions of Non-activated Esters via a Product-base Mechanism
- Yamashita, Y.; Suzuki, H.; Kobayashi, S.
- Org. Biomol. Chem. 10, 5750 (2012). DOI: 10.1039/C2ob25522g
Development of catalytic asymmetric direct-type 1,4-addition reactions of simple amides and esters
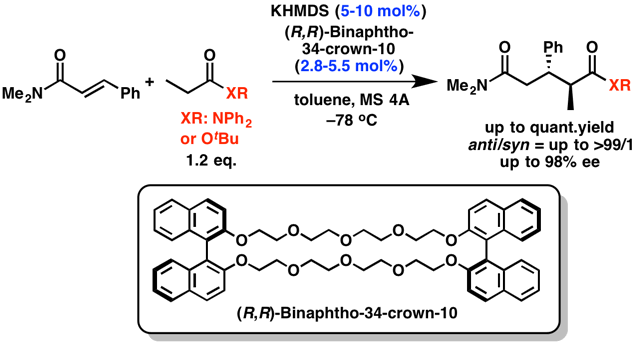
Comment
The development of catalytic asymmetric direct-type reactions of less acidic carbonyl compounds, such as amides and esters, is a challenging topic in organic chemistry. We developed the asymmetric direct 1,4-addition reactions of simple amides with α,β-unsaturated carbonyl compounds by using a catalytic amount of a novel chiral catalyst consisting of a potassium base and a macrocyclic chiral crown ether. The desired 1,5-dicarbonyl compounds were obtained in high yields with excellent diastereo- and enantioselectivities. This is the first example of a highly enantioselective catalytic direct-type reaction of simple amides and esters.
Access to paper
- Catalytic Asymmetric Direct-Type 1,4-Addition Reactions of Simple Amides
- Suzuki, H.; Sato, I.; Yamashita, Y.; Kobayashi, S.
- J. Am. Chem. Soc. 137, 4336 (2015). DOI: 10.1021/jacs.5b01943