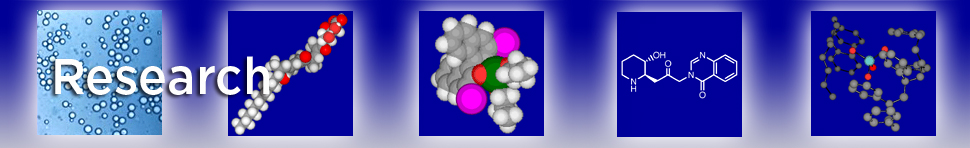
Asymmetric conjugate addition
Asymmetric conjugate addition is a practical and efficient strategy in chemical synthesis, however, controlling the region- and stereo-selectivity still remain a significant challenge. Our group has developed a new effective catalyst, combining Cu(II) and a chiral bipyridine ligand in water, which was successfully applied in the enantioselective conjugate addition. The desired products were obtained in high yields and high enantioselectivities.
Topics
- Asymmetric boron conjugate addition in water
- From conventional Cu(I)-based chemistry to Cu(II)-based chemistry
- Homogeneous vs. heterogeneous catalyst
- Asymmetric silyl conjugate addition in water
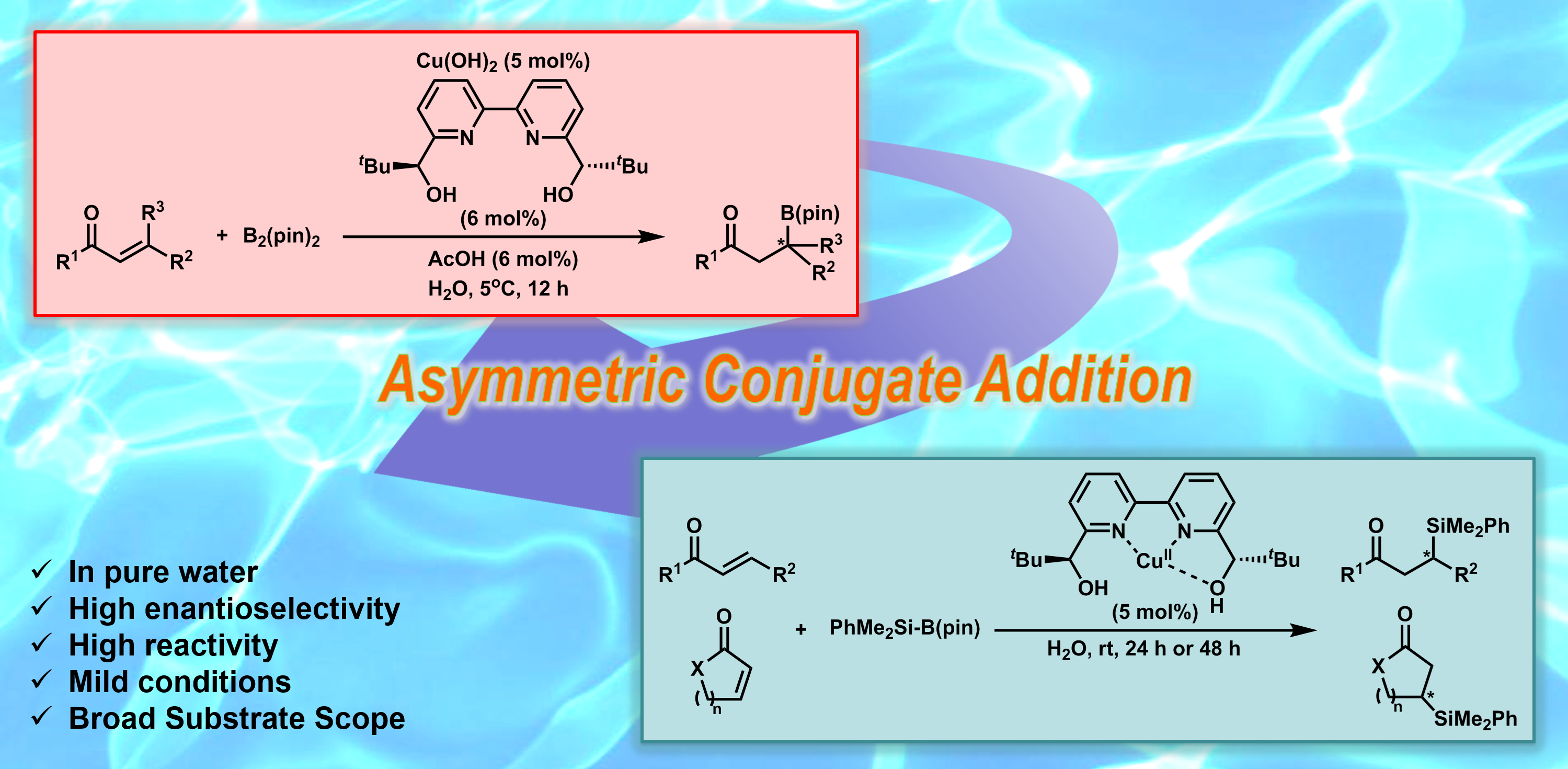
Asymmetric boron conjugate addition in water
Comment
In recent years, optically active organoboranes have attracted considerable attraction as versatile synthons for the synthesis of biologically interesting compounds and of other materials. Enantioselective boron conjugate addition to a,b-unsaturated carbonyl and related compounds provides one of the most efficient routes to α-chiral boron compounds. Previously, reactions have been carried out predominantly by using Cu(I) with a chiral ligand in organic solvents. In contrast, we reported the first example of a chiral Cu(II)-catalyzed asymmetric boron additions in water. Under the optimized conditions, a wide substrate scope was successfully explored, including α,β- or α,β,γ,δ-unsaturated carbonyl compounds, α,β-unsaturated imines and nitriles.
Access to paper
- Chiral Copper(II)-Catalyzed Enantioselective Boron Conjugate Additions to α,β-Unsaturated Carbonyl Compounds in Water
- Kobayashi, S.; Xu, P.; Endo, T.; Ueno, M.; Kitanosono, T.
- Angew. Chem. Int. Ed. 51, 12763-12766 (2012). DOI: 10.1002/anie.201207343
- back to top
From conventional Cu(I)-based chemistry to Cu(II)-based chemistry

Comment
Cu(I)-based chemistry has flourished over the last decade because of the reliable use of species such as soft acids. However, the unique nature of Cu(II) catalysts allows the well-documented Cu(I)-based chemistry to be extended. We have explored a Cu(II)-based strategy instead of using conventional Cu(I)-based chemistry led to an increase in the range of α,β-unsaturated acceptors that are amenable to catalytic asymmetric β-borylation. The ready availability of Cu(II) species, coupled with the ease of operating the reaction in the absence of a strong base, underscores the value of the Cu(II)-based catalyst over an array of well-documented Cu(I)-based catalysts.
Access to paper
- Cu(II)-Based Strategy for Catalytic Enantioselective β-borylation of α,β-unsaturated acceptors
- Zhu, L.; Kitanosono, T.; Xu, P.; Kobayashi, S.
- Chem. Commun. 51, 11685-11688 (2015). DOI: 10.1039/C5CC04295J back to top
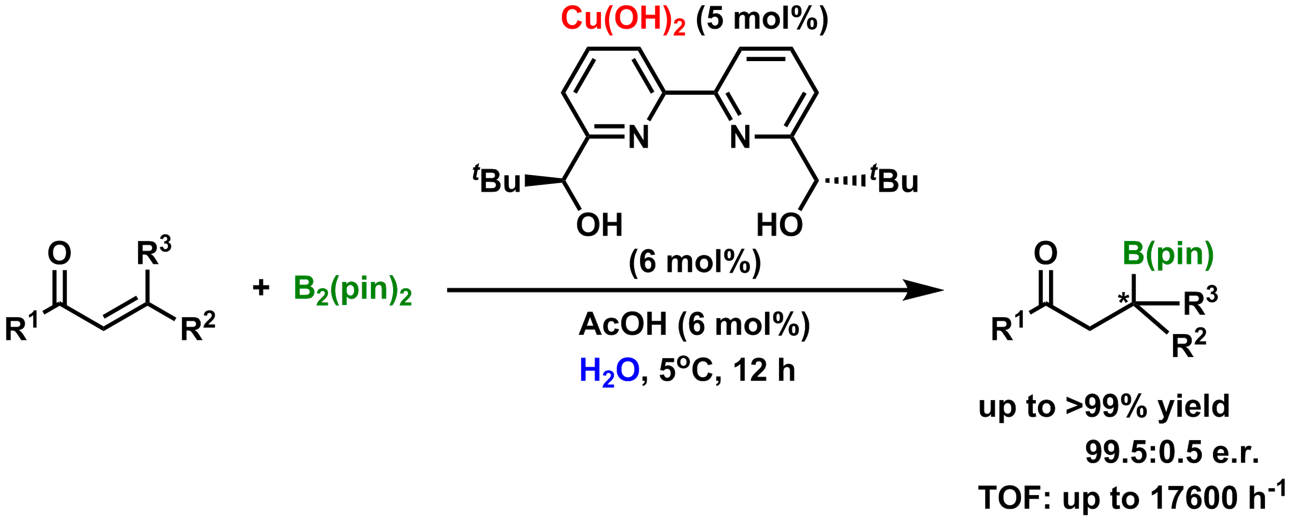
Homogeneous vs. heterogeneous catalyst
Comment
It was proved that a judicious choice of counter anion played a prominent role in Cu(II) catalysis for enantioselective boron conjugate additions in water; the use of Cu(OH)2 renders heterogeneous catalysis, whereas Cu(OAc)2 renders homogeneous catalysis. After testing 27 α,β-unsaturated carbonyl compounds and an α,β-unsaturated nitrile compound, we found that homogeneous catalyst has shown high yields and enantioselectivities for almost all substrates. Meanwhile, heterogeneous catalyst also gave high yields and enantioselectivities with some substrates and the highest TOF (43200 h-1) for an asymmetric conjugate-addition reaction of boron. Further development has elucidated that the catalyst systems were also applicable to the conjugate addition of boron to α,β,γ,δ-unsaturated carbonyl compounds. In addition, cyclic dienones underwent a remarkable switch of regioselectivity between 1,4- and 1,6-modes of the additions through these catalyses.
Access to paper
- Heterogeneous and Homogeneous Chiral Cu(II) Catalysis in Water: Enantioselective Boron Conjugate Additions to Dienones and Dienoesters
- Kitanosono, T.; Xu, P.; Kobayashi, S.
- Chem. Commun. 49, 8184-8186 (2013). DOI: 10.1039/C3CC44324H
- Asymmetric Boron Conjugate Additions to Enones in Water Catalyzed by Copper(0)
- Kitanosono, T.; Kobayashi, S.
- Asian J. Org. Chem. 2, 961-966 (2013). DOI: 10.1002/ajoc.201300201
- Cu(II)-Catalyzed Asymmetric Boron Conjugate Addition to α,β-Unsaturated Imines in Water
- Kitanosono, T.; Xu, P.; Isshiki, S.; Zhu, L.; Kobayashi, S.
- Chem. Commun. 50, 9336-9339 (2014). DOI: 10.1039/C4CC04062G
- Heterogeneous versus Homogeneous Copper(II) Catalysis in Enantioselective Conjugate-Addition Reactions of Boron in Water
- Kitanosono, T.; Xu, P.; Kobayashi, S.
- Chem. Asian. J. 9, 179-188 (2014). DOI: 10.1002/asia.201300997
- Chiral Cu(II)-Catalyzed Enantioselective β-borylation of α,β-unsaturated Nitriles in Water
- Zhu, L.; Kitanosono, T.; Xu, P.; Kobayashi, S.
- Beilstein J. Org. Chem. 11, 2007-2011 (2015). DOI: 10.3762/bjoc.11.217
Asymmetric silyl conjugate addition in water
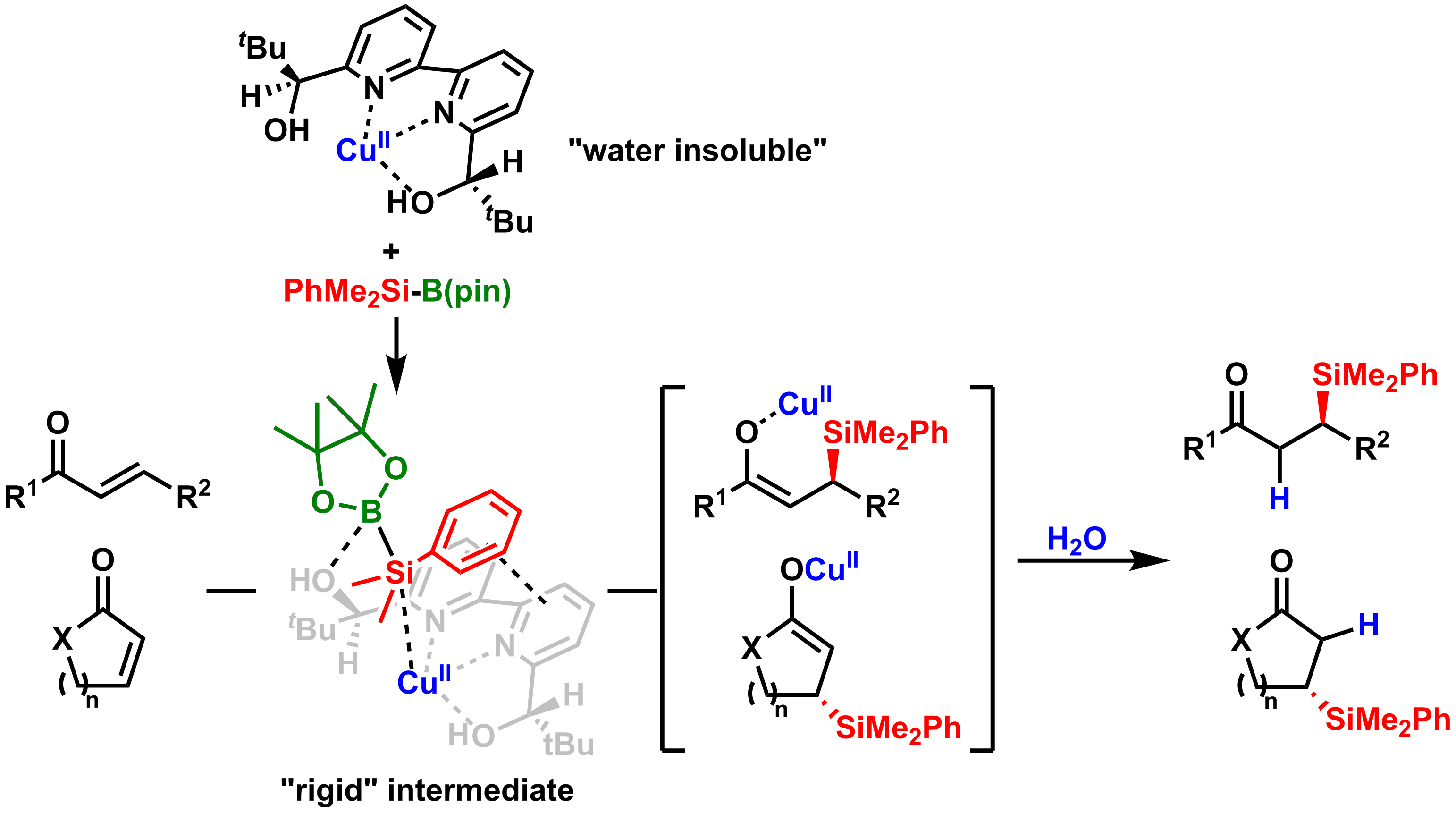
Comment
Acicular purplish crystals were obtained from Cu(acac)2 and a chiral bipyridine ligand. Although the crystals were not soluble, they nevertheless catalyzed asymmetric silyl conjugate addition of lipophilic substrates in water. Indeed, the reactions proceeded efficiently only in water; they did not proceed well either in organic solvents or in mixed water/organic solvents in which the catalyst/substrates were soluble. This is in pronounced contrast to conventional organic reactions wherein the catalyst/substrates tend to be in solution. Several advantages of the chiral Cu(II) catalysis in water over previously reported catalyst systems have been demonstrated. Water is expected to play a prominent role in constructing and stabilizing sterically confined transition states and accelerating subsequent protonation to achieve high yields and enantioselectivities.
Access to paper
- An Insoluble Copper(II) Acetylacetonate-Chiral Bipyridine Complex that Catalyzes Asymmetric Silyl Conjugate Addition in Water
- T. Kitanosono,§ L. Zhu,§ C. Liu, P. Xu, S. Kobayashi (§ contributed equally)
- J. Am. Chem. Soc ASAPDOI: 10.1021/jacs.5b11418