Publication List (2016)
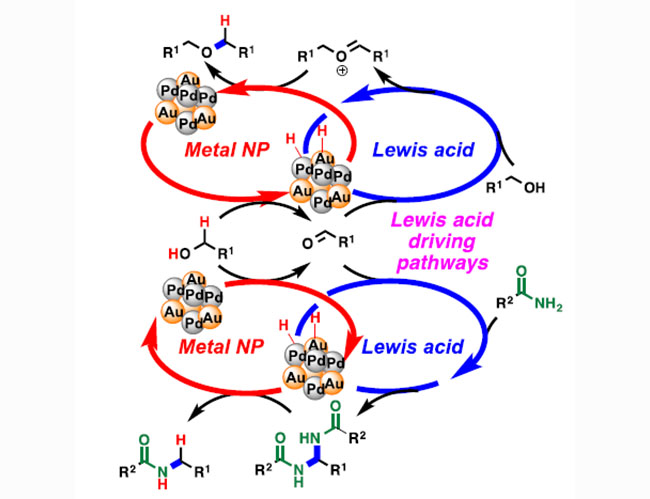
Lewis acid-driven reaction pathways in synergistic cooperative catalysis over gold%2Fpalladium bimetallic nanoparticles for hydrogen autotransfer reaction between amide and alcohol
H. Miyamura, S. Isshiki, H. Min, S. Kobayashi
Chinese J. Catal., 37, 1662-1668 (2016). DOI: 10.1016/S1872-2067(16)62483-X
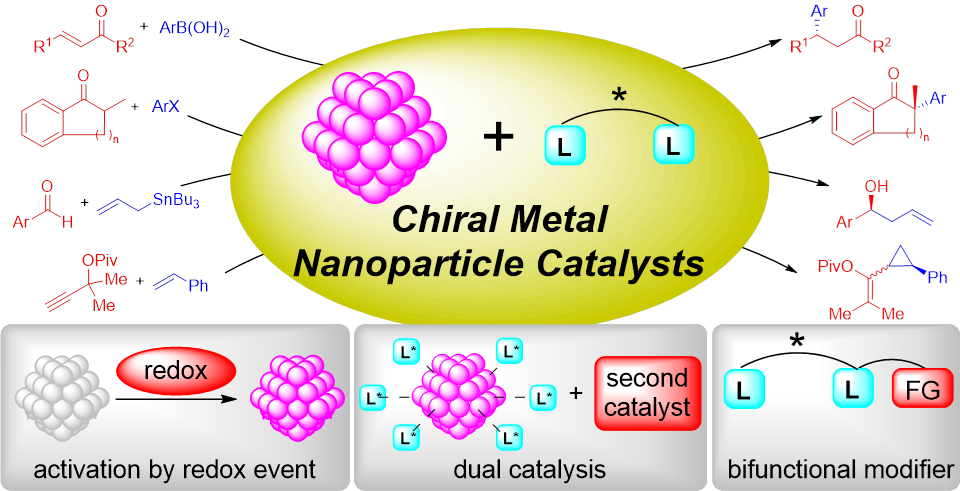
Chiral Ligand-Modified Metal Nanoparticles as Unique Catalysts for Asymmetric CC Bond-Forming Reactions: How Are Active Species Generated?
T. Yasukawa, H. Miyamura and S. Kobayashi
ACS Catal. , 6, 7979-7988(2016). DOI: 10.1021/acscatal.6b02446
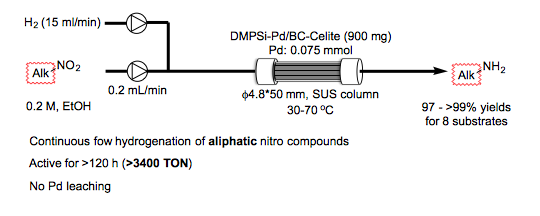
Catalytic Hydrogenation of Aliphatic Nitro Compounds with Polysilane/Bone Charcoal-Supported Palladium Catalysts under Continuous-Flow Conditions
Y. Saito, H. Ishitani, S. Kobayashi
Asian JOC , 5, 1124-1127 (2016). DOI: 10.1002/ajoc.201600279
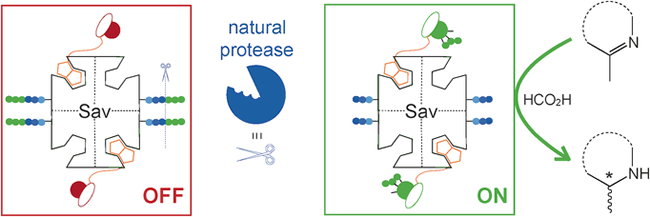
Upregulation of an Artificial Zymogen by Proteolysis
Z. Liu, V. Lebrun, T. Kitanosono, H. Mallin, V. Köhler, D. Häussinger, D. Hilvert, S. Kobayashi, T. R. Ward
Angew. Chem. Int. Ed. , 55, 11587-11590 (2016). DOI: 10.1002/anie.201605010
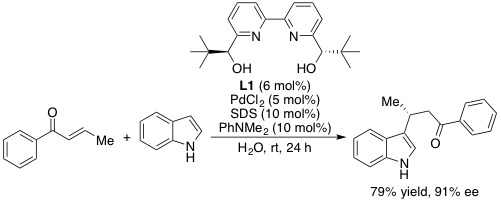
Surfactant-aided chiral palladium(II) catalysis exerted exclusively in water for the C–H functionalization of indoles
T. Kitanosono, M. Miyo, S. Kobayashi
ACS Sustainable Chem. Eng. 4, 6101-6106(2016).DOI: 10.1021/acssuschemeng.6b01519
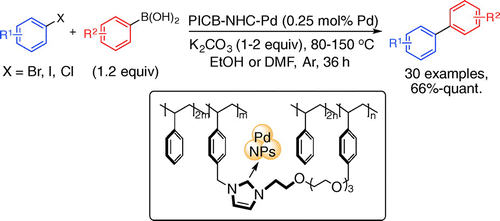
N-Heterocyclic Carbene Coordinated Heterogeneous Pd Nanoparticles as Catalysts for Suzuki–Miyaura Coupling
H. Min, H. Miyamura, S. Kobayashi
Chem. Lett.45, 837-839 (2016).DOI: 10.1246/cl.160369
Development of a Simple Adjustable Zinc Acid/Base Hybrid Catalyst for C-C and C-O Bond-Formation and C-C Bond-Cleavage Reactions
Y. Yamashita, K. Minami, Y. Saito, S. Kobayashi
Chem. Asian J.11, 2372-2376(2016).DOI: 10.1002/asia.201600682
Catalytic Asymmetric Direct-type 1,4-Addition Reactions of Simple Esters
I. Sato, H. Suzuki, Y. Yamashita, S. Kobayashi
Org. Chem. Front., 3, 1241-1245 (2016).DOI: 10.1039/C6QO00242K
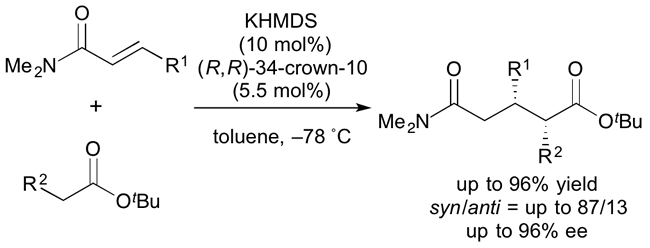
Development of Chiral Metal Amides as Highly Reactive Catalysts for Asymmetric [3 + 2] Cycloadditions
Y. Yamashita, S. Yoshimoto, M. J. Dutton, S. Kobayashi
Beilstein J. Org. Chem., 12, 1447-1452 (2016). DOI: 10.3762/bjoc.12.140
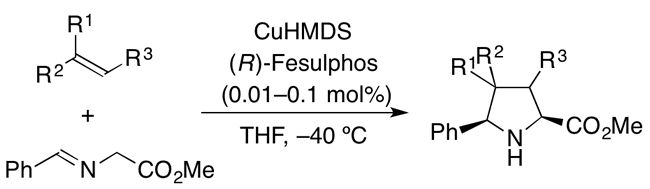
Asymmetric Arylation of Imines Catalyzed by Heterogeneous Chiral Rhodium Nanoparticles
T. Yasukawa, T. Kuremoto, H. Miyamura, S. Kobayashi
Org. Lett.18, 2716-2718 (2016). DOI: 10.1021/acs.orglett.6b01172
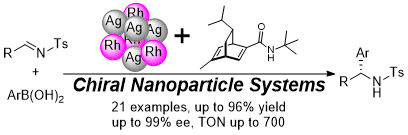
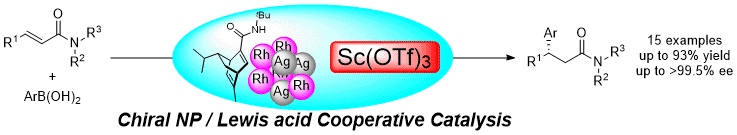
Chiral Nanoparticles/Lewis Acids as Cooperative Catalysts for Asymmetric 1,4-Addition of Arylboronic Acids to α,β-Unsaturated Amides
T. Yasukawa, Y. Saito, H. Miyamura, S. Kobayashi
Angew. Chem., Int. Ed. Accepted DOI: 10.1002/anie.201601559
Enantioselective Organometallic Catalysis in Flow
H. Ishitani, Y. Saito, S. Kobayashi
Topics in Organometallic Chemistry , 57, 213-218 (2016). URL
Flow “Fine” Synthesis: High Yielding and Selective Organic Synthesis by Flow Methods
S. Kobayashi
Chem. Asian J., 11 , 425-426 (2016). DOI: 10.1002/asia.201500916
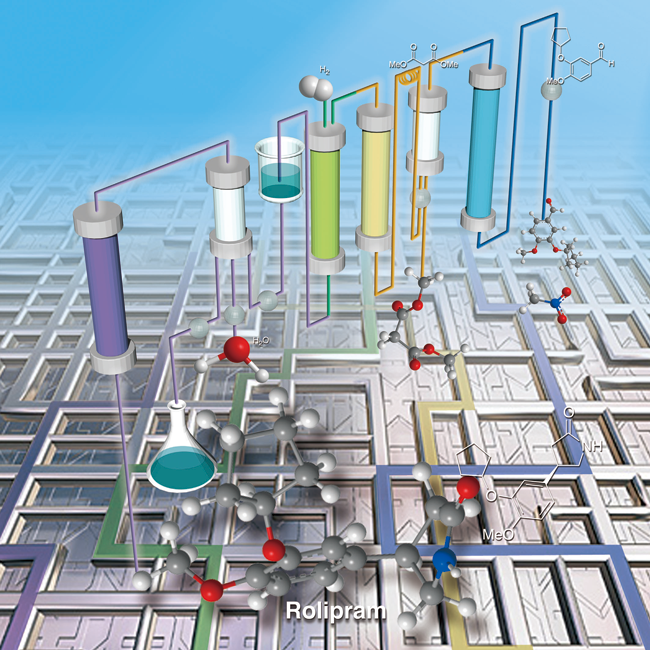
Synthesis of Nitro-Containing Compounds through Multistep Continuous Flow with Heterogeneous Catalysts
H. Ishitani, Y. Saito, T. Tsubogo, S. Kobayashi
Org. Lett., 18, 1346-1349 (2016). DOI: 10.1021/acs.orglett.6b00282
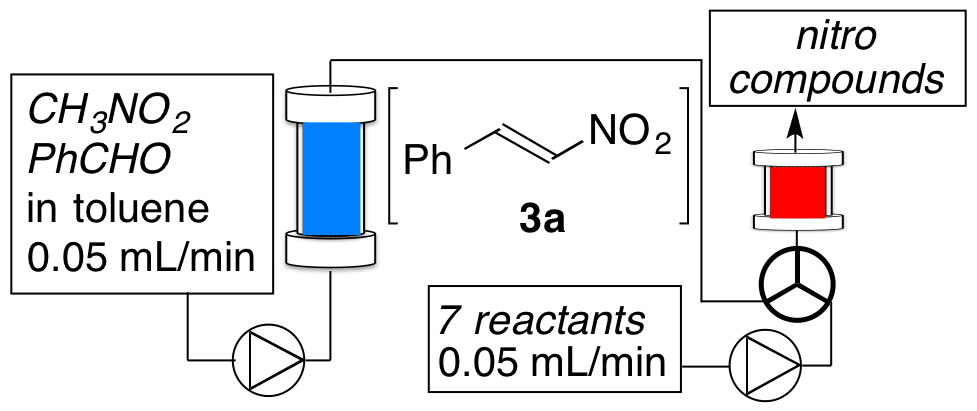
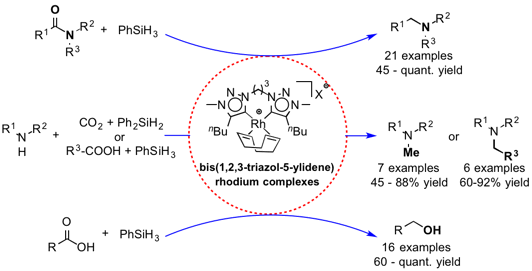
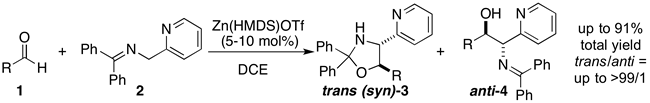
Chelating Bis(1,2,3-triazol-5-ylidene) Rhodium Complexes: Versatile Catalysts for Hydrosiylation Reactions
T. V. Q. Nguyen, W.-J. Yoo, S. Kobayashi
Adv. Synth. Catal. 358 , 452-458 (2016). DOI: 10.1002/adsc.201500875