Asymmetric synthesis using chiral metal nanoparticles
Asymmetric transformations are essential reactions to construct basic skeletons of useful compounds such as medicines and pesticides. However, most catalytic asymmetric synthesis requires novel metal based homogeneous catalysts that have problems such as difficulty of recovery and contamination into the product. Therefore, it is important subject to develop highly active, selective, recoverable and reusable heterogeneous asymmetric catalysts since we can save the use of precious resource and reduce waste.
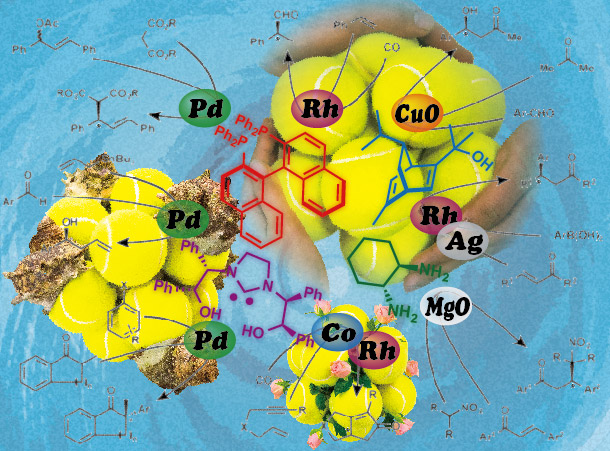
We also developed asymmetric synthesis using chiral ligand modified polymer-incarcerated metal nanoparticle catalyst, so-called, heterogeneous chiral metal nanoparticle catalysts. Chiral metal nanoparticle catalysts showed high activity and robustness and we found the characteristic nature of chiral nanoparticle catalyst that is distinct to the nature of the corresponding chiral homogeneous metal complex catalyst.
Review:
- Chiral Metal Nanoparticle-Catalyzed Asymmetric C–C Bond Formation Reactions
- T. Yasukawa, H. Miyamura, S. Kobayashi
- Chem. Soc. Rev. 43, 1450-1461 (2014). DOI: 10.1039/c3cs60298b
Topics
- Chiral Rh nanoparticle catalyzed asymmetric 1,4-addition reactions
- Cellulose supported chiral Rh nanoparticle catalyst
Chiral Rh nanoparticle catalyzed asymmetric 1,4-addition reactions
Comment
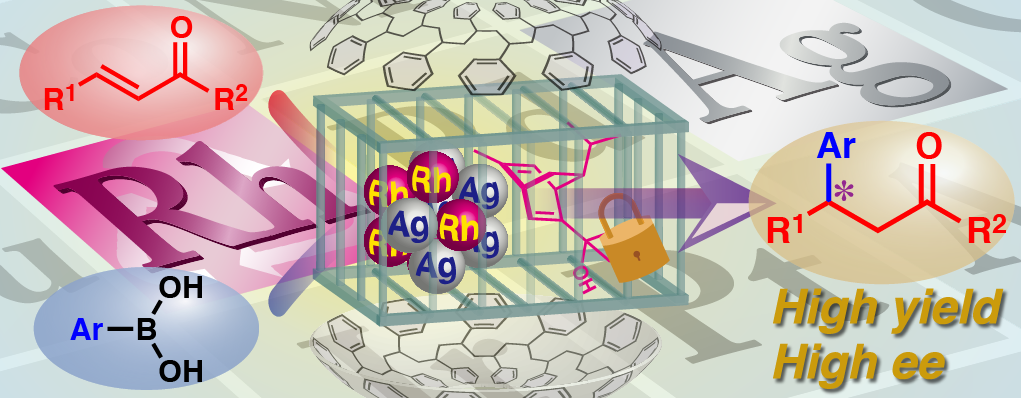
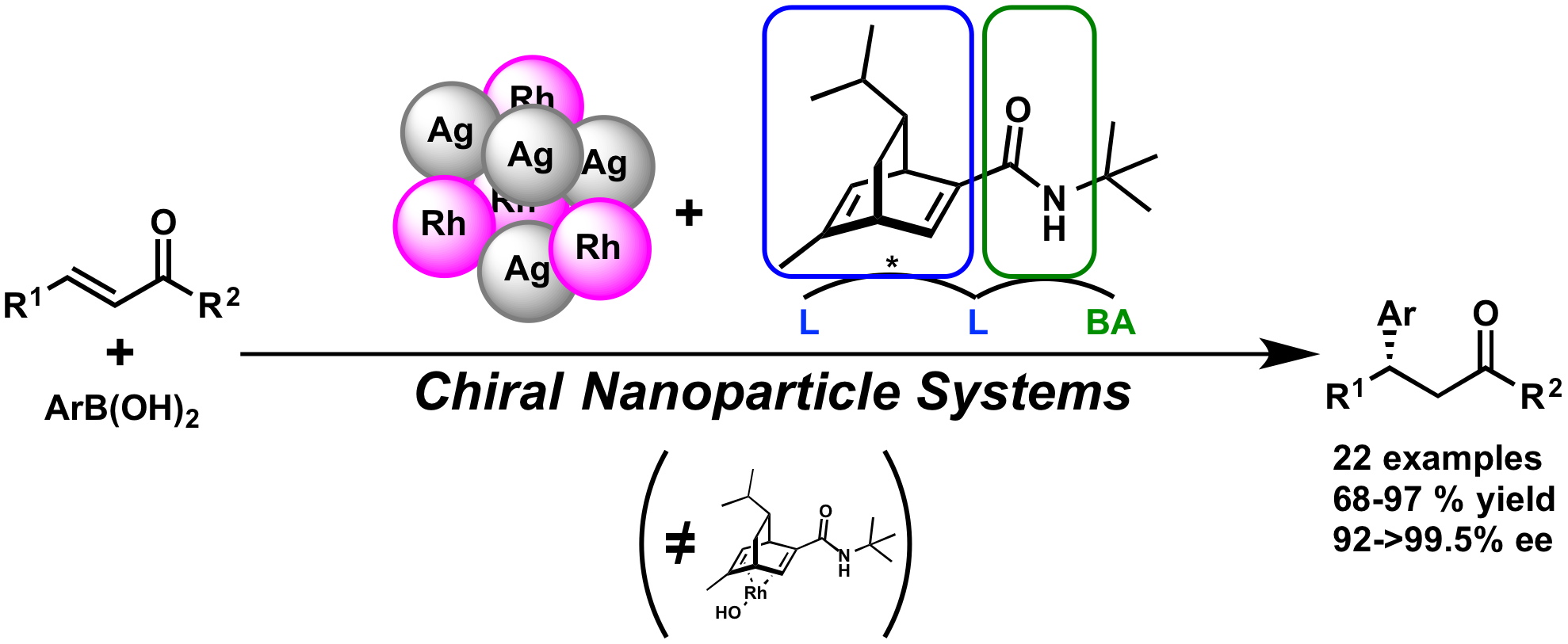
We found that in the presence of chiral diene ligand, polymer-incarcerated Rh nanoparticle could catalyze asymmetric 1,4-addition of arylboronic acids to enones in high yield and high enantioselectivity without the metal leaching from support. The catalytic activity was improved by using Rh/Ag bimetallic catalyst and the wide substrate generality was demonstrated. The catalyst could be recovered and reused without significant loss of activity.
Moreover, by introduction of chiral diene bearing secondary amide moiety, high yields and outstanding enantioselectivities were also achieved in asymmetric 1,4-addition to unsaturated esters. This ligand is assumed to be a bifunctional ligand that can not only coordinate to metals but also activate substrates by the amide part. Several mechanistic studies such as reaction profiles and non-linear effect analyses revealed that the heterogeneous nanoparticle system showed different behavior from the corresponding homogeneous metal complex system and specific nature of active species was realized.
Access to paper
- Polymer-Incarcerated Chiral Rh/Ag Nanoparticles for Asymmetric 1,4-Addition Reactions of Arylboronic Acids to Enones: Remarkable Effects of Bimetallic Structure on Activity and Metal Leaching
- T. Yasukawa, H. Miyamura, S. Kobayashi
- J. Am. Chem. Soc. 134, 16963-16966 (2012). DOI: 10.1021/ja307913e
- Chiral Metal Nanoparticle Systems as Heterogeneous Catalysts beyond Homogeneous Metal Complex Catalysts for Asymmetric Addition of Arylboronic Acids to α,β-Unsaturated Carbonyl Compounds
- T. Yasukawa, A. Suzuki, H. Miyamura, K. Nishino, S. Kobayashi
- J. Am. Chem. Soc. 137, 6616-6623 (2015). DOI: 10.1021/jacs.5b02213
- back to top
Cellulose supported chiral Rh nanoparticle catalyst
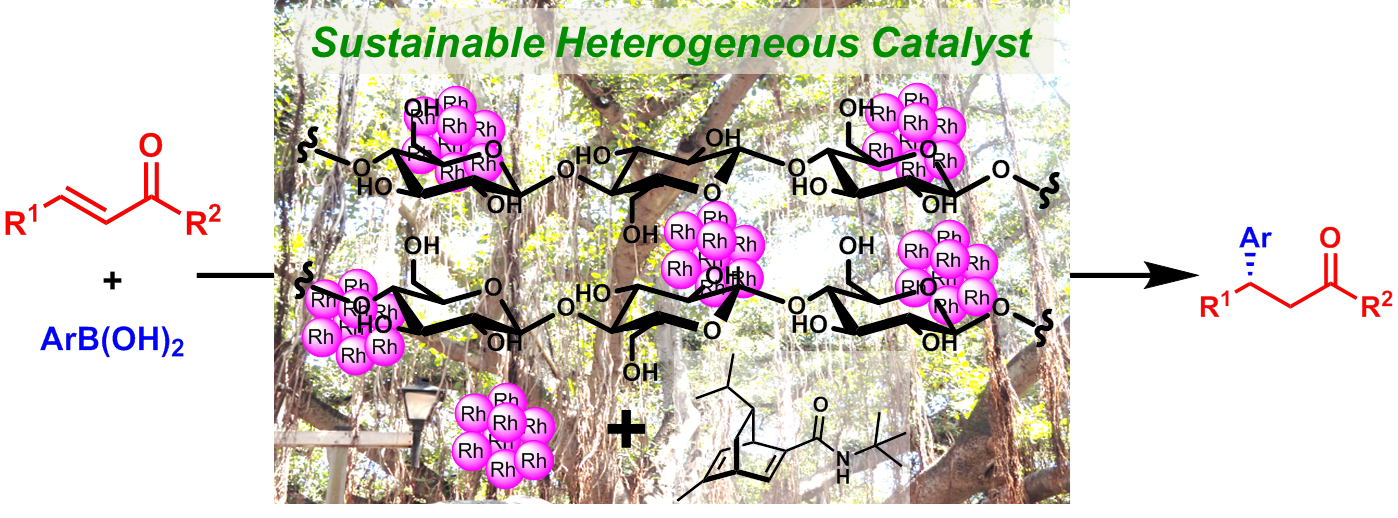
Comment
We developed cellulose, which is abundant in nature, supported chiral Rh nanoparticle catalyst to establish green and sustainable asymmetric catalyst system. In contrast to polymer supported catalyst, Rh nanoparticle dispersed well on cellulose without dopant of silver and the obtained catalyst showed high activity and excellent enantioselectivity in asymmetric 1,4-addition of arylboronic acids to enones and unsaturated esters. Moreover, we conducted SR-MAS analysis (swollen resin–magic angle spinning, a kind of solid state NMR that utilizing a solvent to swell the sample) using the specific pulse sequence and the results suggested that the ligand adsorbed on the Rh nanoparticle was selectively observed.
Access to paper
- Cellulose-Supported Chiral Rh Nanoparticles as Sustainable Heterogeneous Catalysts for Asymmetric Carbon-Carbon Bond-Forming Reactions
- T. Yasukawa, H. Miyamura, S. Kobayashi
- Chem. Sci. 6, 6224-6229 (2015). DOI: 10.1039/C5SC02510A
- back to top