Development of catalytic reactions using organosuperbases
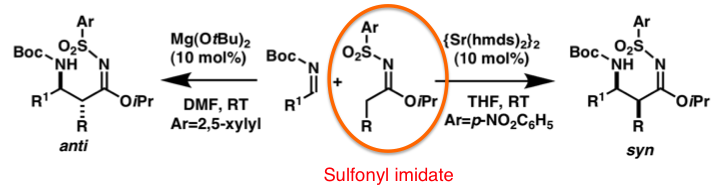
Our group has developed new reagents for organic synthesis. We found that sulfonylimidates prepared from nitriles and sulfonamides could react with several electrophiles to form the desired products in high yields with high stereoselectivities. In the above scheme, an interesting example of controlling the diastereoselection of the products by simply switching reaction conditions is shown. In addition, we have developed phosphonylimidates as analogues of sulfonylimidates, for similar catalytic reactions with DBU as a catalyst. Although we have investigated the use of DBU as a catalyst for the Mannich reactions of sulfonylimidates, DBU is not a reactive catalyst and a much more active catalyst was desired. During the course of this study, we found that organobases including a P atom on their structure, phosphazenes and phosphatoranes, which are called as organosuperbases, showed higher catalyst activity, and high yields and high selectivities were obtained when iBu-PAP was employed as a catalyst.
Topics
- Development of catalytic direct-type Mannich reactions of sulfonylimidates using an organosuperbase as a catalyst
- Development of catalytic direct-type 1,4-addition reactions of sulfonylimidates using an organosuperbase as a catalyst
- Catalytic formation of heterocycles via cooperative activation of substrates by organosuperbases and water
Development of catalytic direct-type Mannich reactions of sulfonylimidates using an organosuperbase as a catalyst
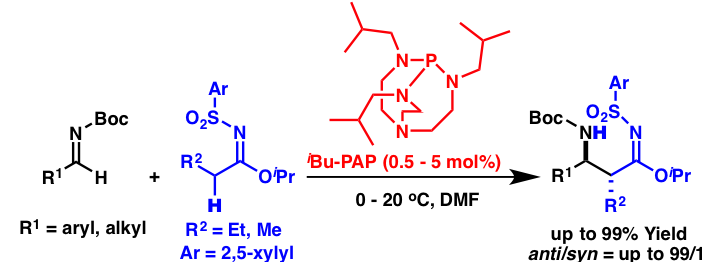
Comment
Although we have investigated the use of DBU as a catalyst for the Mannich reactions of sulfonylimidates, DBU is not a reactive catalyst and a much more active catalyst was desired. During the course of this study, we found that organosuperbases showed higher catalyst activity than DBU, and high yields and high selectivities were obtained when iBu-PAP was employed as a catalyst. A mechanistic study indicated that iBu-PAP worked as an initiator in this catalytic reaction and that the desired reaction proceeded through a “product base mechanism.” In this mechanism, the reaction intermediate itself worked as the real base for the deprotonation of next substrate, and no catalyst (organosuperbase) regeneration occurred during the catalytic cycle.
Access to paper
- Highly Efficient Organosuperbase-Catalyzed Mannich-type Reactions of Sulfonylimidates with Imines: Successful Use of Aliphatic Imines as Substrates and a Unique Reaction Mechanism
- Nakano, J.; Masuda, K.; Yamashita, Y.; Kobayashi, S.
- Angew. Chem. Int. Ed. 51, 9525 (2012). DOI: 10.1002/anie.201204572
- back to top
Development of catalytic direct-type 1,4-addition reactions of sulfonylimidates using an organosuperbase as a catalyst
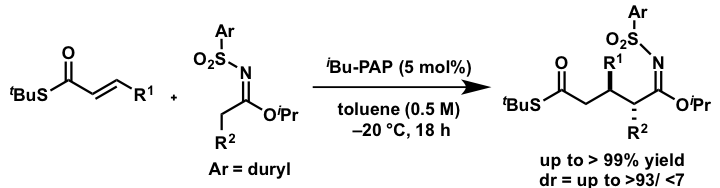
Comment
We developed catalytic direct-type 1,4-addition reactions of sulfonylimidates using iBu-PAP as a catalyst. Despite successful examples of catalytic direct-type 1,4-addition reactions using enolate precursors bearing no EWG group on their α-positions, such examples are limited, and reactions using sulfonylimidates as enolate precursors is promising. The desired reactions proceeded smoothly in high yields with high diastereoselectivities using α,β-unsaturated thioester as a 1,4-addition acceptors.
Access to paper
- Organosuperbase-catalyzed Direct-type Michael Addition Reactions of Sulfonylimidates as Ester Surrogates
- Masuda, K.; Nakano, J.; Yamashita, Y.; Kobayashi, S.
- Asian J. Org. Chem. 2, 303 (2013). DOI: 10.1002/ajoc.201300014
- back to top
Catalytic formation of heterocycles via cooperative activation of substrates by organosuperbases and water
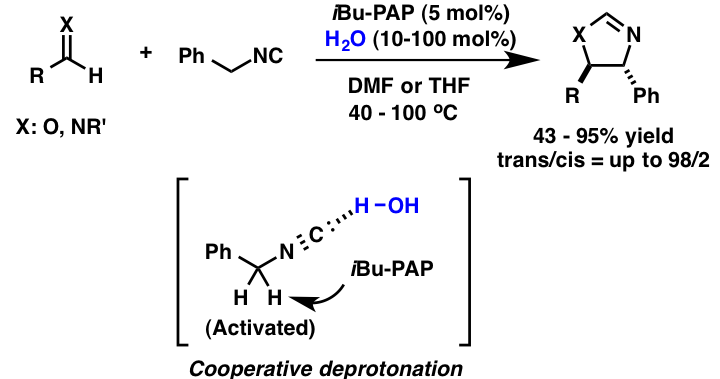
Comment
Interestingly, we observed a cooperative effect between organosuperbases and water in the catalytic cyclization of benzyl isocyanate with aldehydes or imines. The iBu-PAP catalyzed reactions were enhanced dramatically by the addition of a small amount of water to the reaction system. NMR studies indicated that water worked as a Brønsted acid for the activation of benzyl isocyanate via hydrogen bonding.
Access to paper
- A cooperative water effect in proazaphosphatrane-catalysed heterocycle synthesis
- Honey A. Mark; Yamashita, Y.; Kobayashi, S.
- Chem. Commun. 50, 3288 (2014). DOI: 10.1039/C3CC49808E
- back to top