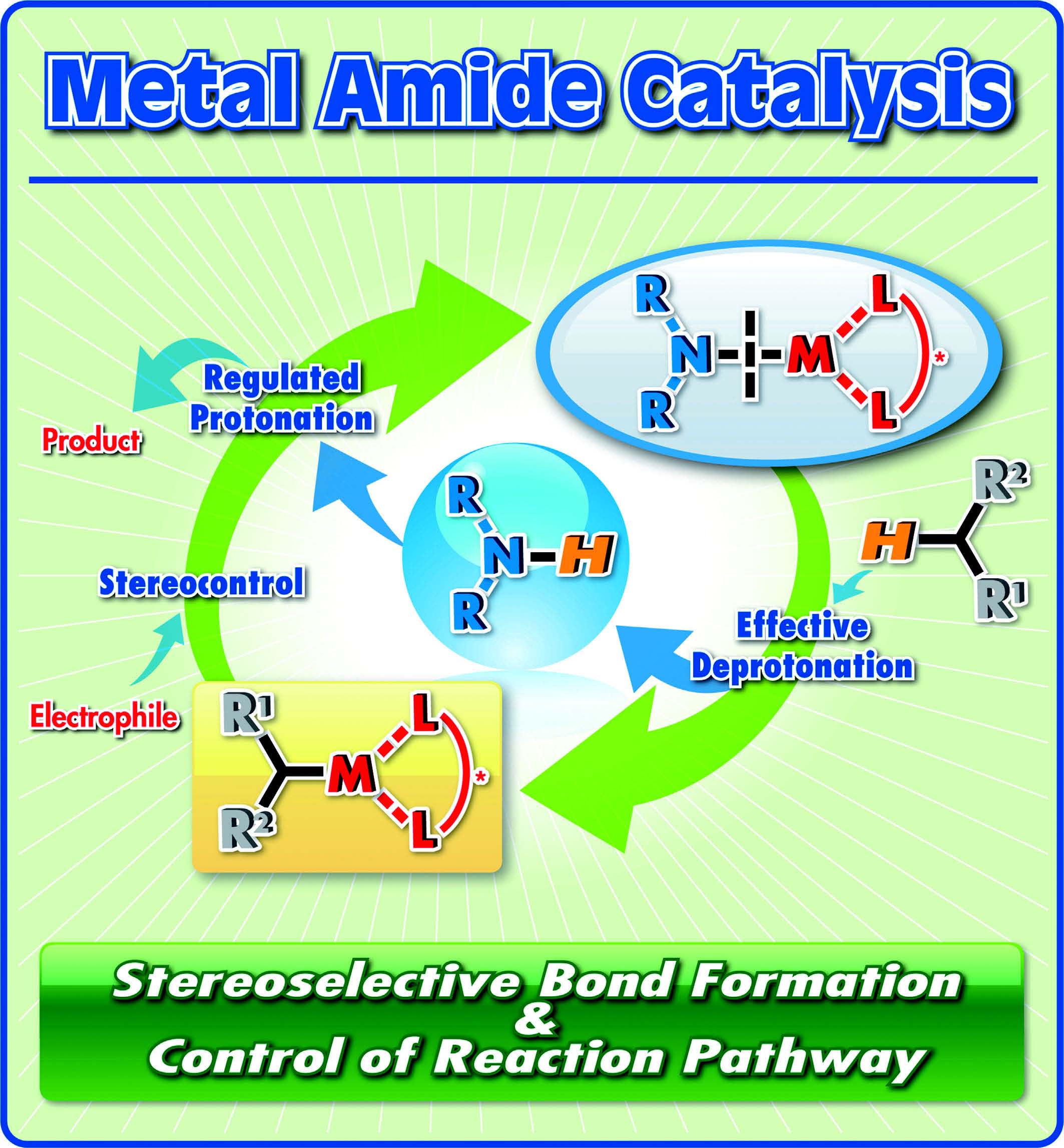
Development of metal amides as catalysts for organic reactions
Metal amides have a metal-nitrogen bond in their structure and possess strong basicity as a nitrogen anion equivalent. Among them, alkaline metal amides such as lithium diisopropylamide (LDA) and potassium hexamethyldisilazide (KHMDS) have been employed as stoichiometric strong bases in organic synthesis. However, metal amides, with the exception alkaline metal amides, have not been employed as catalysts in highly stereoselective carbon-carbon bond forming reactions. Currently we are focusing on the development of metal amides as simple cooperative acid/base catalysts with stronger basicity, which bear closed acidic and basic parts on the same molecule, in stereoselective carbon-carbon bond forming reactions.
Review:
- Metal Amides as the Simplest Acid/Base Catalysts for Stereoselective Carbon–Carbon Bond-Forming Reactions,
- Y. Yamashita, S. Kobayashi,
- Chem. Eur. J., 19, 9420-9427(2013). DOI: 10.1002/chem.201300908
Topics
- Catalytic asymmetric [3+2] cycloaddition reactions of α-aminophosphonate Schiff bases
- Catalytic asymmetric [3+2] cycloaddition reactions of α-aminoester Schiff bases using chiral silver amide catalyst
- Catalytic asymmetric Mannich-type reactions using chiral copper amide catalyst
- Catalytic asymmetric [3+2] cycloaddition reactions of azomethine imines with terminal alkynes using a chiral copper amide catalyst
- Development of Lewis acid/metal amide hybrid catalyst
- Development of catalytic allylation reactions using zinc amide as a catalyst
Catalytic asymmetric [3+2] cycloaddition reactions of α-aminophosphonate Schiff bases
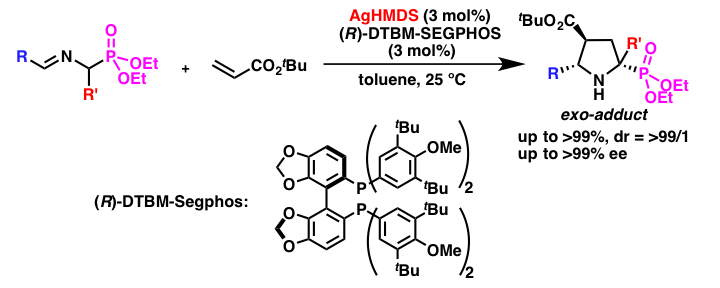
Comment
Catalytic asymmetric [3+2] cycloaddition reactions of α-aminophosphonate Schiff bases are an important method to prepare phosphorous analogues of highly substituted proline derivatives. However, it had been assumed that α-aminophosphonate Schiff bases are poor substrates in catalytic asymmetric reactions due to the less acidic nature of their α-hydrogens. We found that the desired [3+2] cycloaddition reactions proceeded with high stereoselectivities by using a chiral silver amide catalyst. This is the first example of a catalytic asymmetric reaction using a chiral silver amide.
Access to papers
- Chiral Silver Amide-catalyzed Enantioselective [3 + 2] Cycloaddition of α-Aminophosphonates with Olefins
- Yamashita, Y.; Guo, X.-X.; Takashita, R.; Kobayashi, S.
- J. Am. Chem. Soc. 132, 3262 (2010). DOI: 10.1021/ja100101n
- Chiral Silver Amides as Effective Catalysts for Enantioselective [3+2] Cycloaddition Reactions
- Yamashita, Y.; Imaizumi, T.; Guo, X.-X.; Kobayashi, S.
- Chem. Asian J. 6, 2550 (2011). DOI: 10.1002/asia.201100246
Catalytic asymmetric [3+2] cycloaddition reactions of α-aminoester Schiff bases using chiral silver amide catalyst
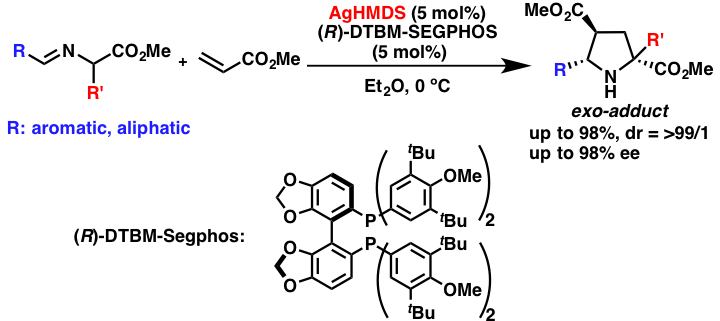
Comment
Chiral silver amides have been successfully applied for catalytic asymmetric [3+2] cycloaddition reactions of α-amino ester Schiff bases. This reaction has been previously performed using several kinds of chiral catalysts; however, successful examples of exo-selective reactions are limited. Furthermore, less reactive α-aminoester Schiff bases prepared from aliphatic aldehydes were also found to be applicable, and the desired reactions proceeded with high stereoselectivities.
Access to papers
- Chiral Silver Amide Catalyst for the [3+2] Cycloaddition of α-Amino Esters to Olefins
- Yamashita, Y.; Imaizumi, T.; Kobayashi, S.
- Angew. Chem. Int. Ed. 50, 4893 (2011). DOI: 10.1002/anie.201008272
- Chiral Silver Amides as Effective Catalysts for Enantioselective [3+2] Cycloaddition Reactions
- Yamashita, Y.; Imaizumi, T.; Guo, X.-X.; Kobayashi, S.
- Chem. Asian J. 6, 2550 (2011). DOI: 10.1002/asia.201100246
Catalytic asymmetric Mannich-type reactions using chiral copper amide catalyst
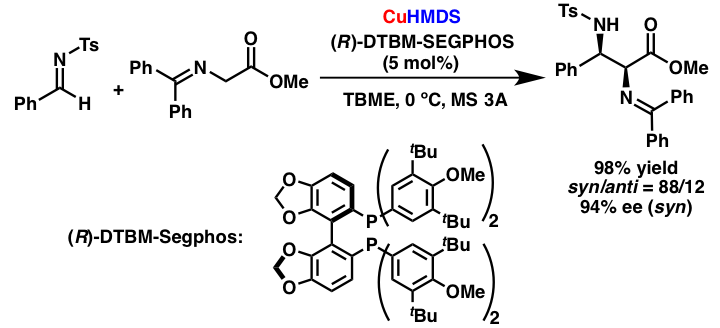
Comment
We also developed catalytic asymmetric Mannich-type reactions of glycine ester Schiff bases with imines by using a chiral copper (I) catalyst. The desired reaction proceeded with high enantioselectivities by using a very bulky ligand, DTBM-Segphos. This is the first example of a highly stereoselective catalytic asymmetric reaction using a chiral copper (I) amide catalyst.
Access to paper
- Chiral Copper Amide Catalyzed Asymmetric Mannich-Type Reactions of Glycine Schiff Bases
- Yamashita, Y.; Yoshimoto, S.; Masuda, K.; Kobayashi, S.
- Asian J. Org. Chem. 1, 327 (2012). DOI: 10.1002/Ajoc.201200092
Catalytic asymmetric [3+2] cycloaddition reactions of azomethine imines with terminal alkynes using a chiral copper amide catalyst
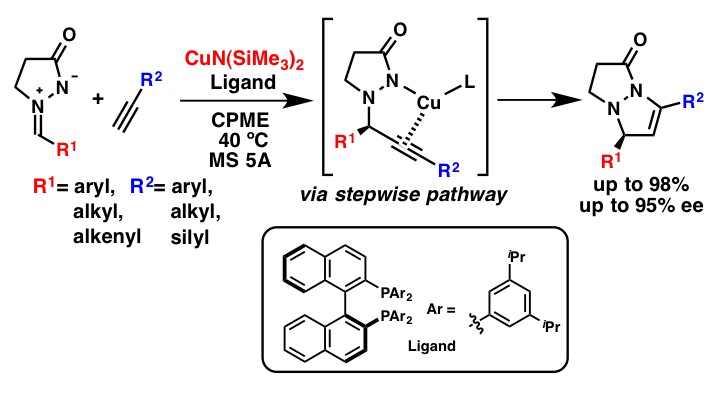
Comment
It was revealed that catalytic [3+2] cycloaddition reactions of azomethine imines with terminal alkynes proceeded in the presence of a silver or a copper amide catalyst to afford bicyclic pyrazolidinones in high yields. Furthermore, by using a chiral bisphosphine ligand, the desired reactions proceeded in high yields with high diastereo- and enantioselectivities. Mechanistic studies indicated that this reaction proceeded through a stepwise pathway (1,2-addition and intramolecular cyclization) and that the less acidic conjugate acid, secondary amine (H-HMDS), played a key role in the catalytic cycle.
Access to paper
- Group 11 Metal Amide-Catalyzed Asymmetric Cycloaddition Reactions of Azomethine Imines with Terminal Alkynes
- Imaizumi, T.; Yamashita, Y.; Kobayashi, S.
- J. Am. Chem. Soc. 134, 20049 (2012). DOI: 10.1021/ja311150n
Development of Lewis acid/metal amide hybrid catalyst
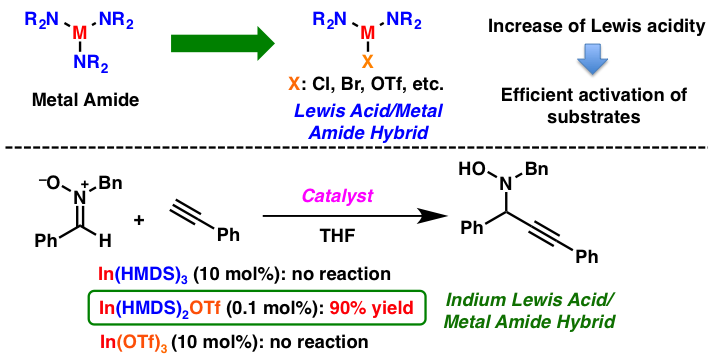
Comment
Lewis acids and metal amides have been employed frequently in organic synthesis as useful acids or bases. However, Lewis acid-base pairs are believed to be inactive due to mutual quenching of their respective reactivities. We have developed a new Lewis acid/metal amide species, in which the metal amide bearing an anionic group with a strong electron-withdrawing nature, could work as an effective acid/base catalyst. When we use indium Lewis acid/metal amide hybrid catalysts, In(HMDS)2X (X = Cl, OTf), the catalytic 1,2-addition reactions of terminal alkynes with nitrones proceeded smoothly. It was found that indium Lewis acid (In(OTf)3) or indium amide (In(HMDS)3) could not catalyze this reaction. We believe that this concept can be applied to generate other acid/base hybrid catalysts for challenging reactions.
Access to paper
- A Lewis acid/metal amide hybrid as an efficient catalyst for carbon–carbon bond formation
- Yamashita, Y.; Saito, Y.; Imaizumi, T.; Kobayashi, S.
- Chem. Sci. 5, 3958 (2014). DOI: 10.1039/C4SC01332H
Development of catalytic allylation reactions using zinc amide as a catalyst
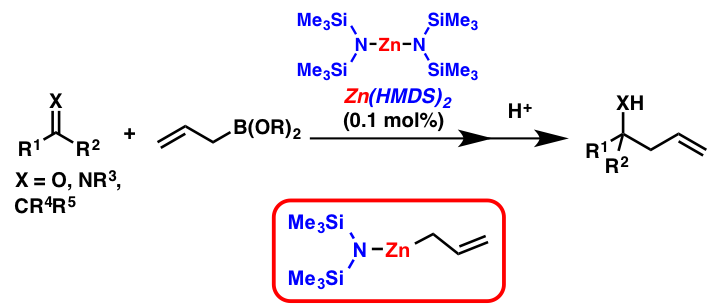
Comment
Metal amides have been employed mainly as a strong Brønsted base in organic synthesis. However, the use of metal amides as effective Lewis acid/Lewis base catalysts is relatively rare. We found that allylation reactions of carbonyl compounds with allylboroate proceeded smoothly by using Zn(HMDS)2 as a catalyst. It was found that the key step is the transmetalation between the zinc amide and allylboronates, and smooth formation of allylzinc species was confirmed by NMR analysis. Furthermore, asymmetric allylation reaction was achieved by using a chiral ligand with the zinc amide catalyst.
Access to papers
- Facile Preparation of Allylzinc Species from Allylboronates and Zinc Amide via a Boron-to-zinc Exchange Process and their Reactions with Carbonyl Compounds, Imines and Hydrazones
- Cui, Y.; Yamashita, Y.; Kobayashi, S.
- Chem. Commun. 48, 10319 (2012). DOI: 10.1039/C2cc34340a
- Catalytic Use of Zinc Amide for Transmetalation with Allylboronates: General and Efficient Catalytic Allylation of Carbonyl Compounds, Imines, and Hydrazones
- Cui, Y.; Li, W.; Sato, T.; Yamashita, Y.; Kobayashi, S.
- Adv. Synth. Catal. 355, 1193 (2013). DOI: 10.1002/Adsc.201201134