Cyclic Luciferase for Protease Imaging
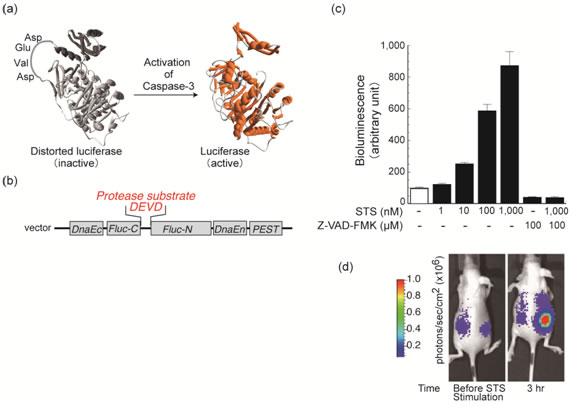
We developed a bioluminescent indicator for high-throughput sensing and noninvasive real-time imaging of caspase activities in living cells and animals. Firefly luciferase connected with a substrate sequence of caspase-3 (Asp-Glu-Val-Asp) is cyclized by an intein DnaE (a catalytic subunit of DNA polymerase III). When the cyclic luciferase is expressed in living cells, the luciferase activity greatly decreases due to a steric effect. If caspase-3 is activated in the cells, it cleaves the substrate sequence embedded in the cyclic luciferase and the luciferase activity is restored. We demonstrated quantitative sensing of caspase-3 activities in living cells upon extracellular stimuli. Furthermore, the indicator enabled noninvasive imaging of the time-dependent caspase-3 activities in living mice. Although we demonstrate the usefulness of the present indicator showing caspase-3 activation, it should be emphasized that the present cyclic luciferase using the protein splicing can be applied to any proteases. With this basic concept, a rapid screening system and a noninvasive imaging technique can be developed for understanding the mechanism of proteolysis and discovering novel chemical compounds modulating protease activities.