Imaging of β-actin mRNA
(Related research:Imaging of mitochondrial mRNA)
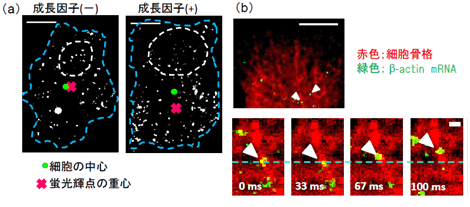
In living cells, movement and localization of mRNAs play an important role in post-transcriptional regulation of gene expression. Such localization of mRNAs in a cytoplasmic region generates cell polarity, resulting cell motility and body segment formation. Single mRNA imaging in live cells is a useful technique to elucidate its precise localization and dynamics. We herein describe a method for visualizing endogenous mRNAs in living cells with single molecule sensitivity using genetically encoded probes. The probe uses a sequence-specific RNA-binding domain, Pumilio homology domain (PUM-HD), and reconstitution of split fragments of enhanced GFP (EGFP). PUM-HD recognizes 8-RNA sequence, and the specificity of PUM-HD for the RNA-base sequences is altered by replacing RNA-recognizing amino acid residues within the PUM-HD elements, so that amino acid mutations in PUM-HD allow for recognition of a specific region in a target mRNA. Each split fragment of EGFP is connected to a PUM-HD mutant that recognizes the specific sequence of the target mRNA. When the probes bind to the target mRNA, the split fragments complement and emit fluorescence. The EGFPs reconstituted on the mRNAs were monitored with a total internal reflection fluorescence (TIRF) microscope. To demonstrate the applicability of this method, we visualized a cytosolical β-actin mRNA in living mammalian cells. We examined the possibility of visualizing β-actin mRNAs in single mRNA sensitivity. The result shows that each fluorescent spot in live cells represented a single β-actin mRNA and that distinct spatial and temporal movement of the individual β-actin mRNA was visualized. We also estimated the average velocity of the movement of the single mRNAs along microtubules in live cells. Our method presents several advantages. One advantage is that a target mRNA is endogenous. Therefore, this method enables us to observe natural movement of the mRNA in live cells. Second, single mRNA imaging is possible to acquire information related to inhomogeneous behavior, kinetics and velocity, of which crucial data are lost in ensemble measurement. A third advantage is the small size of the probes. This method succeeded in visualizing single mRNA with a single fluorescent protein because the fluorescence complementation and TIRF microscopy reduce the background fluorescence signals. Visualization with a small tag is preferred because the tags would change the natural conformation of a target mRNA and prevent formation of mRNA-protein complexes. These advantages in the present method ensure that our method is widely applicable to tracking various mRNAs of interest in the native state of living cells with single-mRNA sensitivity.