Reaction Pathway of the Conjugate Addition of Lithium
Organocuprate Clusters to Acrolein
Eiichi Nakamura*,Ā Seiji Mori,Ā and Keiji Morokuma**
J. Am. Chem. Soc., 119, 4900-4910 (1997)
This article contains 3D-pictures. In order to see these moving
pictures, you need to have a Chemscape
Chimie plug-in in your Netscape Navigator plug- in folder.
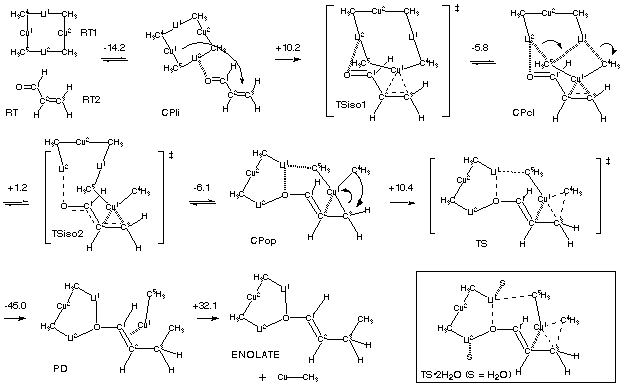
The reaction pathway of transfer of a methyl group of a cuprate
cluster (Me2CuLi)2 to acrolein (conjugate addition of the methyl
group) has been studied with the hybrid density functional B3LYP
method. In addition to two previously proposed species, a
lithium/carbonyl coordination complex (CPli) and a copper/olefin
complex retaining a closed cuprate structure (CPcl), a new
copper/olefin complex with an open cuprate structure (CPop) was
characterized and proven to be an intermediate directly leading to
the conjugate addition product (PD) via a TS of C-C bond formation
(TS). The overall pathway of the reaction can be viewed, in one way,
as C-C bond formation via reductive elimination of a Cu(III)
species, and, in another, as a 1,4-addition of MeLi assisted by
copper. The present studies revealed that the large cluster framework
of (Me2CuLi)2 allows intricate cooperation of two lithium atoms and a
copper atom. Mechanistic relationships between conjugate addition,
carbocupration, and alkylation reactions of cuprate are discussed.
A close similarity has been found between the conjugate addition
and the carbocupration of acetylene for the cooperative action of
metal and the involvement of a Cu(III) species.
Return
to 3D Structures of Organometallic reaction Pathway
ü@