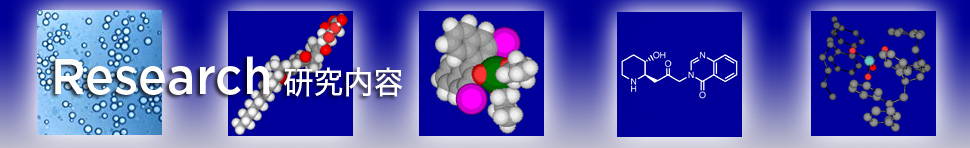
金ナノ粒子触媒による空気中の酸素を酸化剤として用いる種々の反応
Since Haruta's discovery of Au nanoparticle catalyzed aerobic oxidation of CO at low temperature, Au nanoparticle catalysis has gotten much attention as green heterogeneous catalyst for aerobic oxidation. We successfully immobilized Au nanoparticles into polystyrene derived organic co-polymer with cross-linking moieties using the polymer incarceration technique (PI). Thus prepared catalysts can be applied to various types of aerobic oxidations. Interesting bimetallic effect and size effect of Au nanoparticles are highlighted.
Topics
- Polymer incarcerated Au nanoparticle (PI-Au) for alcohol oxidation
- Remarkable bimetallic effect in aerobic oxidation of alcohols
- Polymer-carbon black nanocomposite (PI/CB) as promising and improved support
- Aldehyde oxidation via acyl radical intermediate catalyzed by PI-Au
- Hydroquinone oxidation catalyzed by PI-Au under base free and ambient conditions
- PI-Au imoiblized microchannel flow reactor for aerobic oxidation of alcohol
- Direct ester formation from alcohols catalyzed by PI-Au
- Size effect of Au nanoparticles: amine oxidation using PI-Au
- Direct amide synthesis from alcohols and amines controlled by bimetallic effect and structure
- Direct imine synthesis from alcohols and amines
- Aerobic oxidation of 1,3-dicarbonyl compounds using PI/CB-Au
- Aerobic oxidation of silyl enol ethers and phenyl boronic acid
- Simple polymer incarcerated Au nanoparticles with aluminate cross-linker for oxidations
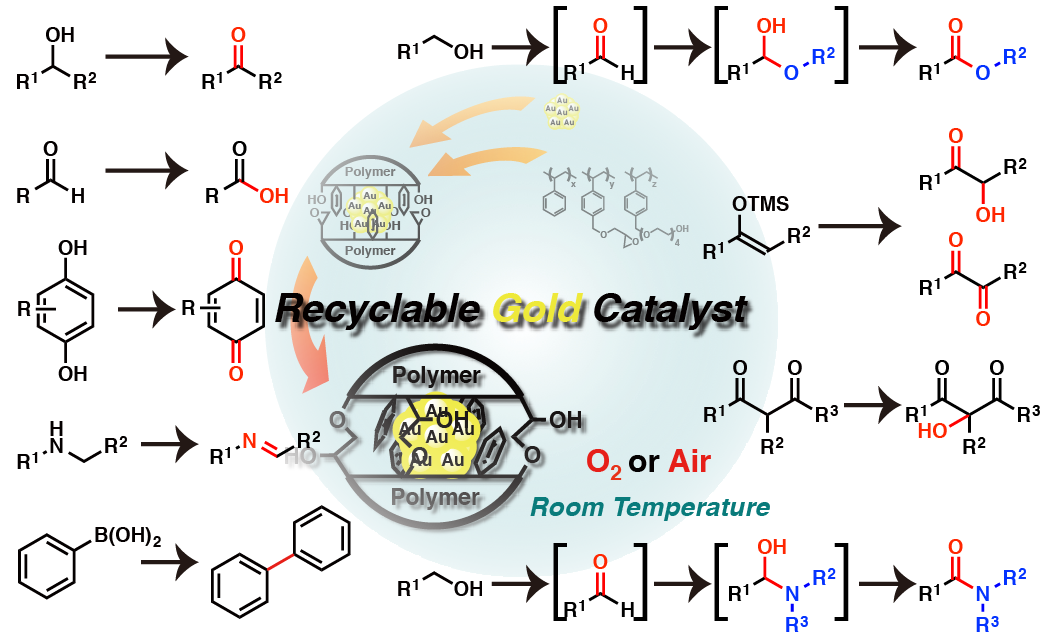
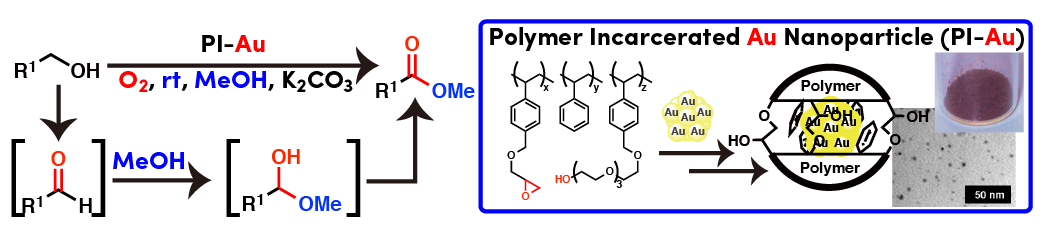
Polymer incarcerated Au nanoparticle (PI-Au) for alcohol oxidation
Comment
Au nanoparticles that were prepared from Au(I) salt and NaBH4 could be incarcerated in polystyrene derived from polymer with cross-linking moieties. Small Au nanoparticles (1 - 4 nm) was immobilized with good size distributions. This heterogeneous catalyst (PI-Au) could be used for aerobic oxidation of alcohol under ambient conditions (atmospheric O2, room temperature, weak basic). PI-Au showed good reusability with keeping high activity.
Access to paper
- Aerobic oxidation of alcohols at room temperature and atmospheric conditions catalyzed by reusable gold nanoclusters stabilized by the benzene rings of polystyrene derivatives
- Miyamura, H.; Matsubara, R.; Miyazaki, Y.; Kobayashi, S.
- Angew. Chem., Int. Ed., 46, 4151-4154 (2007). DOI: 10.1002/anie.200700080 [VIP]
- Selected as inside cover
- back to top
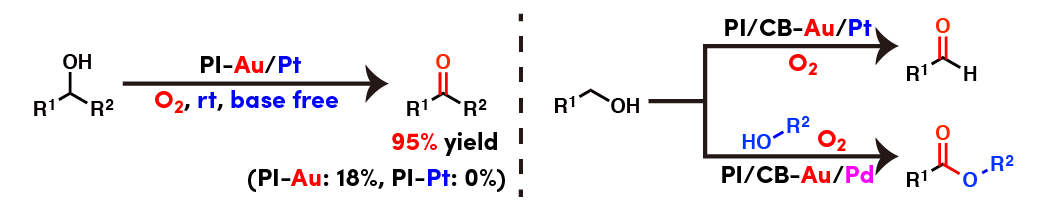
Remarkable bimetallic effect in aerobic oxidation of alcohols
Comment
Polymer incarcerated bimetallic nanoparticle catalysts (PI-M1/M2) could be prepared by simply reducing mixture of two metal salts during catalyst preparation. Remarkably enhanced activity of Au/Pt bimetallic nanoparticle catalyst could be observed for alcohol oxidation under base free conditions compared with single metal nanoparticle catalysts.
We also found that Au/Pt nanoparticle catalyst showed excellent activity for oxidation of primary alcohol to aldehyde, on the other hand, Au/Pd nanoparticle catalyst showed good activity for direct oxidative esterification from two different alcohols.
Access to papers
- Gold-platinum bimetallic clusters for aerobic oxidation of alcohols under ambient conditions
- Miyamura, H.; Matsubara, R.; Kobayashi, S.
- Chem. Commun., 2031-2033 (2008). DOI: 10.1039/b800657a
- Remarkable Effect of Bimetallic Nanocluster Catalysts for Aerobic Oxidation of Alcohols: Combining Metals Changes the Activities and the Reaction Pathways to Aldehydes/Carboxylic Acids or Esters
- Kaizuka, K.; Miyamura, H.; Kobayashi, S.
- J. Am. Chem. Soc., 132, 15096-15098 (2010). 10.1021/ja108256h
- back to top
Polymer-carbon black nanocomposite (PI/CB) as promising and improved support
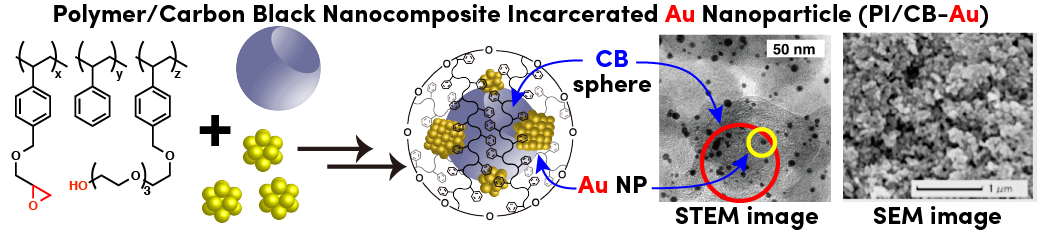
Comment
Metal loading and catalytic activity were enhanced employing nanocomposite of polymer with cross-linking moieties and hollow center spherical carbon black (~ 45 nm). Thus prepared polymer incarcerated carbon stabilized Au nanoparticle catalyst (PI/CB-Au) could be used aerobic oxidation of alcohols under mild conditions. Small Au nanoparticles (1-3 nm) and spherical nanocomposite capsules (~55 nm) were observed by STEM and SEM analyses.
Access to paper
- Aerobic oxidation of alcohols under mild conditions catalyzed by novel polymer-incarcerated, carbon-stabilized gold nanoclusters
- Lucchesi, C.; Inasaki, T.; Miyamura, H.; Matsubara, R.; Kobayashi, S
- Adv. Synth. Catal., 350,1996-2000 (2008). DOI: 10.1002/adsc.200800319
- back to top
Aldehyde oxidation via acyl radical intermediate catalyzed by PI-Au
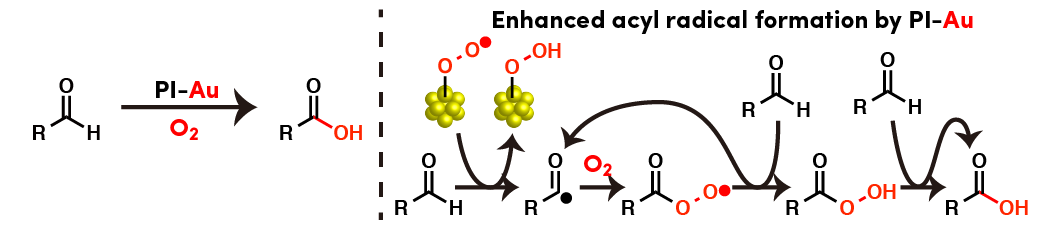
Comment
PI-Au could be applied to aerobic oxidation of an aldehyde to a carboxylic acid. EPR spectroscopy and spin-trapping experiments suggested a radical pathway involving formation of acyl radicals initiated by Au nanoparticles activated by molecular oxygen.
Access to paper
- Enhanced acyl radical formation in the Au nanoparticle-catalysed aldehyde oxidation
- Conte, M.; Miyamura, H.; Kobayashi, S.; Chechik, V.
- Chem. Commun., 46, 145-147 (2010). DOI: 10.1039/b918200d
- back to top
Hydroquinone oxidation catalyzed by PI-Au under base free and ambient conditions
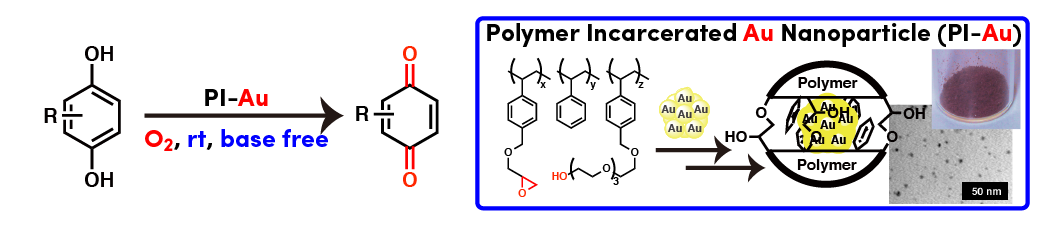
Comment
Polymer incarcerated Au nanoparticle catalyst (PI-Au) could be successfully applied to aerobic oxidation of hydroquinone to corresponding quinone cleanly. Ambient conditions (room temperature, atmospheric air or oxygen, base free) can be employed.
Access to paper
- Polymer incarcerated gold catalyzed aerobic oxidation of hydroquinones and their derivatives
- Miyamura, H.; Shiramizu, M.; Matsubara, R.; Kobayashi, S.
- Chem. Lett. 37, 360 (2008). DOI: 10.1246/cl.2008.360
- back to top
A Gold-Immobilized Microchannel Flow Reactor for Oxidation of Alcohols with Molecular Oxygen
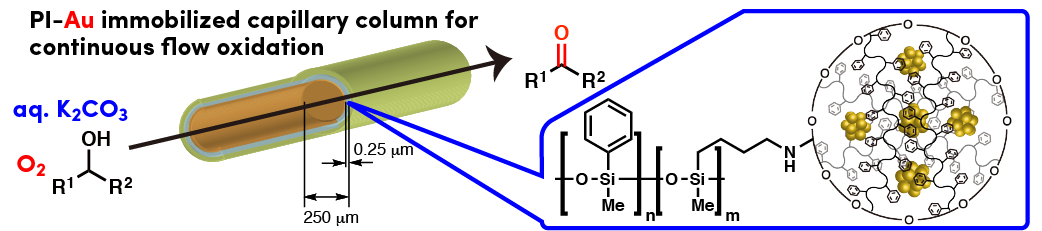
Comment
Polymer incarcerated Au nanoparticle (PI-Au) could be fixed onto a surface of capillary that was simply modified from commercially available GC column. Continuous flow oxidation of alcohol using atmospheric oxygen could proceed successfully using this gold nanoparticle immobilized capillary.
Access to paper
- A Gold-Immobilized Microchannel Flow Reactor for Oxidation of Alcohols with Molecular Oxygen
- Wang, N.; Matsumoto, T.; Ueno, M.; Miyamura, H.; Kobayashi, S.
- Angew. Chem., Int. Ed. 48, 4744 (2009). DOI: 10.1002/anie.200900565
- back to top
Direct ester formation from alcohols catalyzed by PI-Au
Comment
Polymer incarcerated Au nanoparticle (PI-Au) could be used for direct oxidative methylester formation from an alcohol in methanol. Reaction proceeded smoothly under ambient conditions and the catalyst could be reused over 10 times. Although PI-Au was sometimes deactivated during recovery by filtration and drying at room temperature, simple heating under neat conditions could revive the catalytic activity.
Access to paper
- Aerobic oxidative esterification of alcohols catalyzed by polymer-incarcerated gold nanoclusters under ambient conditions
- Miyamura, H.; Yasukawa, T.; Kobayashi, S.
- Green Chem. 12, 776 (2010). DOI: 10.1039/b926877d
- back to top
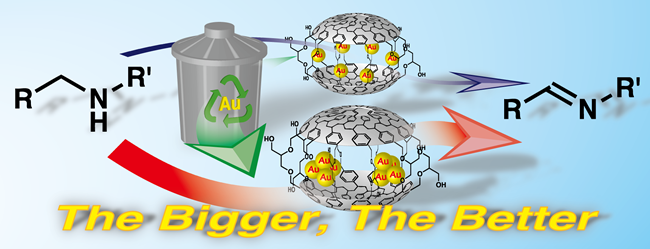
Size effect of Au nanoparticles: amine oxidation using PI-Au
Comment
It is widely accepted that gold nanoparticle showed higher activity when its size was reduced to around 1.5-3 nm. We applied our PI-Au catalyst for aerobic oxidation of amines to imines under atmospheric oxygen at high temperature (110-150 ºC). We unexpectedly found that recovered catalyst showed higher activity than original one during recovery and reuse of PI-Au. Careful structural analysis revealed size of Au nanoparticles increased during reaction. We also prepared catalysts containing bigger nanoparticles by different modified procedures and we confirmed definite correlation between size of Au nanoparticles and reactivity in amine oxidation. This is a quite rare example that bigger Au nanoparticle showed high activity than smaller one.
Access to paper
- Aerobic Oxidation of Amines Catalyzed by Polymer-Incarcerated Au Nanoclusters: Effect of Cluster Size and Cooperative Functional Groups in the Polymer
- Miyamura, H.; Morita, M.; Inasaki, T.; Kobayashi, S.
- Bull. Chem. Soc. Jpn. 84, 588 (2011). DOI: 10.1246/bcsj.20100300 [BCSJ Award Article]
- Selected as cover
- back to top
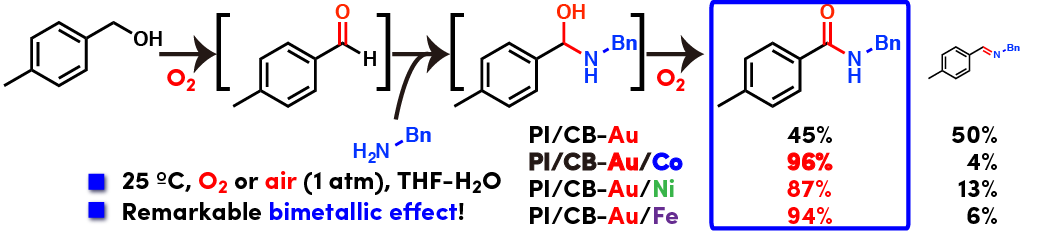
Direct amide synthesis from alcohols and amines controlled by bimetallic effect and structure
Comment
Direct oxidative amide synthesis from alcohols and amines was achieved with bimetallic nanoparticle catalysts of Au and Fe group metals (Fe, Ni, Co) using molecular oxygen as oxidant. Interestingly, Au and Fe/Ni/Co did not form alloyed nanoparticle but separated nanoparticles within same polymer matrix. Selectivity toward amide over imine highly depended on the combination of metal species and morphology of nanoparticles (Please see oxidative imine synthesis from the same combination of substrates). Especially, Au/Co bimetallic nanoparticles showed outstanding performance in this amide synthesis. The reaction proceeded well under mild conditions (room temperature and atmospheric air or oxygen) and wide variety of substrates was applicable. Direct use of aqueous ammonia, aliphatic alcohols, heteroaromatic alcohols and protecting group free amino acids highlights the power of this heterogeneous catalysis.
Access to papers
- Powerful Amide Synthesis from Alcohols and Amines under Aerobic Conditions Catalyzed by Gold or Gold/Iron, -Nickel or -Cobalt Nanoparticles
- Soulé, J.-F.; Miyamura, H.; Kobayashi, S.
- J. Am. Chem. Soc. 133, 18550 (2011). DOI: 10.1021/ja2080086
- back to top
- Direct Amidation from Alcohols and Amines through a Tandem Oxidation Process Catalyzed by Heterogeneous-Polymer-Incarcerated Gold Nanoparticles under Aerobic Conditions
- Soulé, J.-F.; Miyamura, H.; Kobayashi, S.
- Chem. Asian J. 8, 2614 (2013). DOI: 10.1002/asia.201300733
- back to top
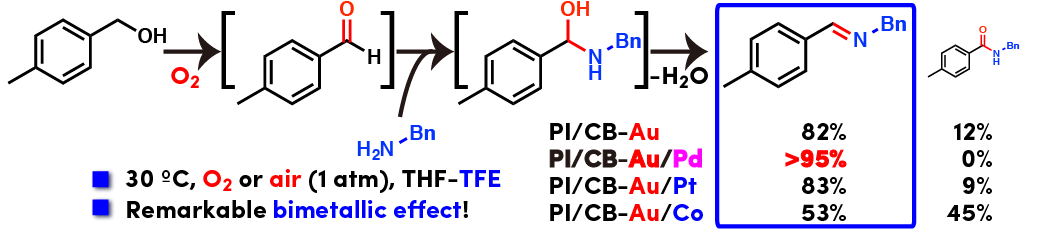
Direct imine synthesis from alcohols and amines
Comment
Direct oxidative imine synthesis from alcohols and amines was achieved with bimetallic nanoparticle catalysts of Au and Pd using molecular oxygen as oxidant. Interestingly, Au and Pd formed alloyed nanoparticle. Selectivity toward amide over imine highly depended on the combination of metal species and morphology of nanoparticles (Please see oxidative amide synthesis from the same combination of substrates). The reaction proceeded well under mild conditions (room temperature and atmospheric air or oxygen) and wide variety of substrates was applicable. Direct use of aqueous aliphatic alcohols, heteroaromatic alcohols and substrates containing unsaturated carbon-carbon bonds highlights the power of this heterogeneous catalysis.
Access to paper
- Selective imine formation from alcohols and amines catalyzed by polymer incarcerated gold/palladium alloy nanoparticles with molecular oxygen as an oxidant
- Soulé, J.-F.; Miyamura, H.; Kobayashi, S.
- Chem. Commun. 49, 355 (2013). DOI: 10.1039/c2cc36213a
- Selected as back cover
- back to top