水溶媒中で展開される高選択的向山アルドール反応
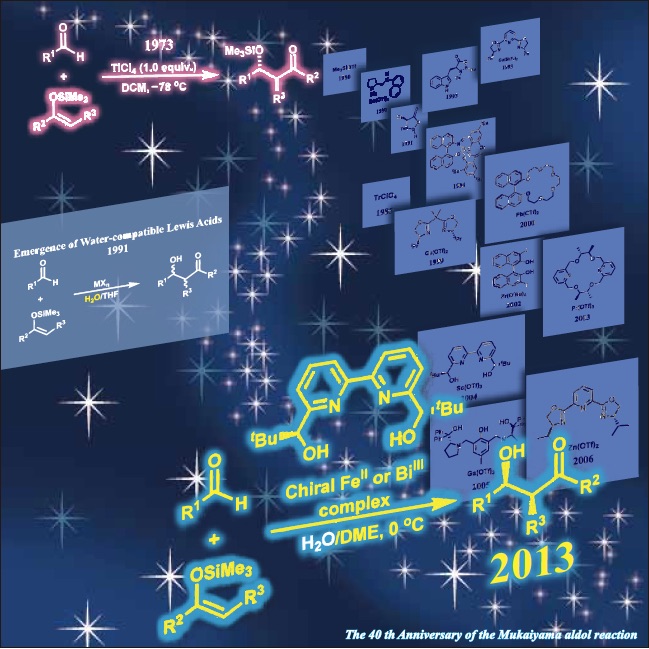
The aldol reaction provides one of the most fundamental and reliable methods for stereoselective C–C bond formation in organic chemistry. Despite its potential versatility, classical aldol reactions performed under basic conditions have suffered from generally low yields and selectivities owing to their reversibility and many competitive side pathways such as dehydration, dimerization, polymerization, and self-condensation. The emergence of TiCl4-mediated aldol reactions of silicon enolates (silyl enol ethers) with aldehydes in 1973—the renowned Mukaiyama aldol reactions—has allowed us to avoid these competitive processes. Being unresponsive towards aldehydes under ambient conditions, silicon enolates react with aldehydes via Lewis acid catalysis. Aldehydes possess more acidic α-protons and are apt to undergo unproductive homo-aldol reactions. In addition, the desired β-hydroxycarbonyl product is more basic than the starting substrates, resulting in a quite low catalyst turnover. Such limitations can be circumvented by combining preformed silicon enolates and Lewis acid catalysis, because the dimerization of aldehydes can be suppressed under mild conditions and the resulting silylated aldol adducts allow for catalyst turnover. The Mukaiyama aldol reaction has thus opened a catalytic enantioselective approach using chiral Lewis acid catalysts.
Reviews:
- Mukaiyama Aldol Reactions in Aqueous Media,
- T. Kitanosono, S. Kobayashi,
- Adv. Synth. Catal., 355, 3095-3118 (2013). DOI: 10.1002/adsc.201300798
- Development of Chiral Catalysts for Mukaiyama Aldol Reactions in Aqueous Media,
- T. Kitanosono, S. Kobayashi,
- Chem. Rec., 14, 130-143 (2014). DOI: 10.1002/tcr.201300040
Topics
水中で安定なLewis酸触媒
Comment
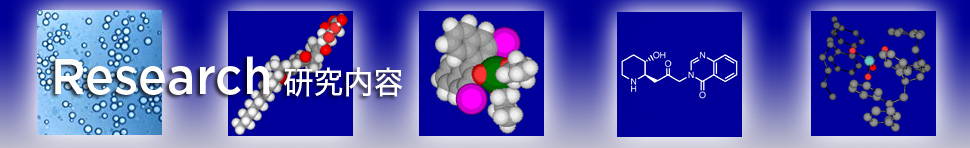
Traditional Lewis acids such as AlIII, TiIV, SnIV, etc. significantly acceralate the Mukaiyama aldol reaction in organic solvents, whereas these Lewis acids decompose rapidly in the presence of water. Indeed, Lewis acids had been believed to be incompatible with water for a long time. In 1991, it was however disclosed that lanthanide trifluoromethanesulfonates (triflates) (Ln(OTf)3) are water-compatible Lewis acids and accelerate the Mukaiyama aldol reaction significantly in aqueous media (water/organic solvents). The conventional problem that Lewis acids decompose in the presence of water was thus overcome using rare-earth triflates (Sc(OTf)3, Y(OTf)3, Ln(OTf)3).
One then wonders why rare-earth triflates, in contrast to conventional Lewis acids, are compatible with water. The Lewis acidity of group 1–15 metal chlorides, perchlorates and triflates was evaluated in a water–THF cosolvent system. In addition to rare-earth metal cations, FeII, CuII, ZnII, CdII, and PbII were disclosed to function as efficient and promising Lewis acids in an aqueous medium. Extensive research enabled us not only to expand the availability of water-compatible Lewis acids but also to establish criteria with which to understand Lewis acidity in an aqueous environment. Given the correlation between metal cations and their catalytic activities, hydrolysis constants (Kh) and exchange rate constants for the substitution of inner-sphere water ligands (water exchange rate constants (WERCs)) were suitable factors for estimating catalytic activities. These active metal compounds were found to have pKh values in the range from about 4.3 (for ScIII) to 10.08 (for CdII) and WERC values greater than 3.2 × 106 M–1s–1. Cations with large pKh values do not generally undergo efficient hydrolysis. In the case of pKh values being less than 4.3, cations are readily hydrolyzed to produce protons in sufficient number to cause rapid decomposition of silicon enolates. On the other hand, when pKh values are higher than 10, Lewis acidities of the cations are too low to catalyze the aldol reaction.
The water-compatible Lewis acid catalysts contributed significantly to an explosive advance in synthetic organic chemistry. Chemists’ interest was then turned toward more challenging objective; stereoselective installation in an aqueous environment. A major difficulty in the construction of asymmetric environments in aqueous conditions is the weakness of noncovalent interactions between substrates, chiral ligands, and metal ions under competitive polar conditions. Conversely, it is anticipated that undesired unselective side reactions can be inhibited and stereochemical regulation stricter than that for other organic solvents can be imposed employing a hydrogen bond network, specific solvation, and hydrophobic interactions, if such processes can be strictly controlled.
Access to papers
- Lanthanide Trifluoromethanesulfonates as Stable Lewis Acids in Aqueous Media. Yb(OTf)3 Catalyzed Hydroxymethylation Reaction of Silyl Enol Ethers with Commercial Formaldehyde Solution
- S. Kobayashi
- Chem. Lett. 2187-2190 (1991). DOI: 10.1246/cl.1991.2187
- Lewis Acid Catalysts Stable in Water. Correlation between Catalytic Activity in Water and Hydrolysis Constants and Exchange Rate Constants for Substitution of Inner-Sphere Water Ligands
- S. Kobayashi, S. Nagayama, T. Busujima
- J. Am. Chem. Soc. , 120, 8287-8288 (1998). DOI: 10.1021/ja980715q
Lewis酸-界面活性剤一体型触媒
Comment
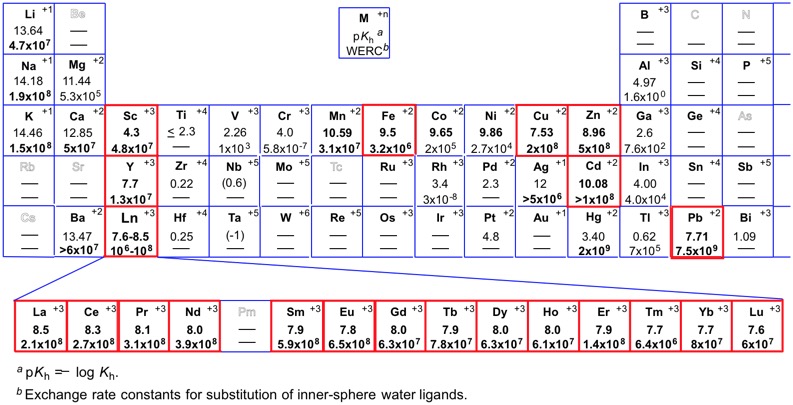
A fundamental challenge of performing the Mukaiyama aldol reactions in water is the competition of rapid hydrolysis of silicon enolates, which can lower reactivity and narrow the substrate generality. To make the desired catalytic pathway dominant over the competitive hydrolysis, a possible approach is to enhance the hydrophobicity of the reaction environments. It was revealed that addition of a catalytic amount of surfactant increased the yield in Sc(OTf)3-catalyzed Mukaiyama aldol reaction in 100% water. Micellar systems containing anionic or nonionic surfactant has led to remarkable enhancement of the reactivity. Sodium dodecylsulfate (SDS) was the most effective. Only cetyltrimethylammonium bromide (CTAB) was found to be an ineffective surfactant due to the possible hydrolysis of the silicon enolate.
A major disadvantage of Lewis acid catalysis aided by surfactants was, nevertheless, the semi-catalytic use of the surfactants required to achieve good results and separation issue. Due to high affinity of water-compatible Lewis acids against water, the relative concentration of the catalyst in the hydrophobic micelles is low. There were, hence, severe substrate limitations in Lewis acid-SDS system; an extremely labile silicon enolates such as cyclohexanone-derived silyl enol ether decomposed rapidly. In 1998, Lewis acid-surfactant combined catalyst (LASC) was emerged as an innovative catalyst, in which a Lewis acid possessed ligands with properties of a surfactant to construct an efficient hydrophobic environment surrounding a Lewis acidic cation.
Access to paper
- Lewis acid catalysis in micellar systems. Sc(OTf)3-Catalyzed aqueous aldol reactions of silyl enol ethers with aldehydes in the presence of a surfactant
- S. Kobayashi, T. Wakabayashi, S. Nagayama, H. Oyamada
- Tetrahedron Lett. , 38, 4559-4562 (1997). DOI: 10.1016/S0040-4039(97)00854-X
- Scandium trisdodecylsulfate (STDS). A new type of lewis acid that forms stable dispersion systems with organic substrates in water and accelerates aldol reactions much faster in water than in organic solvents
- S. Kobayashi, T. Wakabayashi
- Tetrahedron Lett. , 39, 5389-5392 (1998). DOI: 10.1016/S0040-4039(98)01081-8
水系溶媒中での高選択的な触媒的不斉アルドール反応
Comment
Recent advances in Mukaiyama aldol reaction in aqueous media led chemists to examine the possibility of asymmetric catalysis in an aqueous environment. Indeed, almost all successful examples of catalytic asymmetric variants reported in organic solvents since 1990 entailed absolutely aprotic anhydrous conditions as well as quite low reaction temperatures (e.g. –78 oC). The emergence of facile, convenient, and environmentally benign methodology without tedious operation is therefore desirable. A major difficulty in achieving asymmetric catalysis in aqueous media is the weakness of noncovalent interactions between substrates, chiral ligands, and metal ions under competitive polar conditions. Conversely, if capable of being highly controlled, chiral induction in aqueous environments is anticipated to inhibit undesired achiral side reactions and to impose stricter stereocontrol than other organic solvents through hydrogen bond network, specific solvation, and hydrophobic interactions. Despite the formidable task of controlling stereochemistry efficiently in an aqueous environment, recent attempts at this challenging endeavor have been successfully achieved. The underlying strategies in common are as below; 1) chiral aqua complex is stable, 2) the replacement of coordinated water molecule by an aldehyde is rapid and facile, and 3) one enantioface of the coordinated aldehyde carbonyl is shielded effectively.
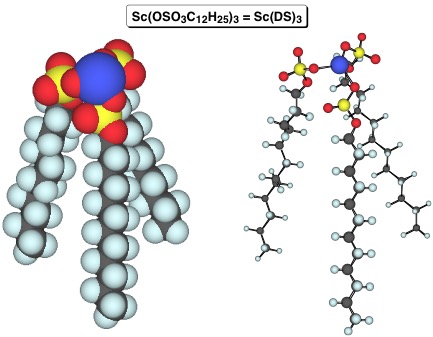
In 2013, forty years since the first advent of asymmetric Mukaiyama aldol reaction, an exhaustive search for a water-compatible Lewis acid could dig up the hidden potentiality of FeII and BiIII, leading to the establishment of broadly applicable and versatile catalytic systems. The ternary catalytic system could enlarge the substrate generality complementarily as the most distinguished catalyst ever reported. The superiority of this methodology over conventional methods has also been demonstrated in terms of high catalytic activity, simplicity of experimental procedures, and a wide substrate range including aqueous aldehydes for which the stereochemistry has been regarded to be difficult to govern. Furthermore, a facile synthesis of the chiral ligand underscores its versatility. The reaction did not proceed at all without use of water. In assumed mechanisms, water plays prominent roles in a) producing the active metal complexes with a high water exchange rate constant (3.2×106) to activate substrates effectively and to catalyze the reaction through a rapid proton transfer in the order of picoseconds, b) facilitating the catalytic turnover with simultaneous desilylation as direct access to aldol adducts or facile recovery of active metal complexes, and c) stabilizing rigid transition states composed of metal complexes and reactants through entropy-driven aggregation derived from the highest cohesive energy density.
In 2015, density functional theory (DFT), combined with the artificial force-induced reaction (AFIR) method, is used to establish the mechanism of FeII-catalyzed reaction. On the bases of the calculations, several thermodynamically stable six- or seven-coordinate complexes in the solution were identified, where the high-spin quintet state is the ground state. Among them, the active intermediates for the selectivity-determining outer-sphere carbon–carbon bond formation are proposed. The multicomponent artificial force-induced reaction (MC-AFIR) method found key transition states for the carbon–carbon bond formation, and explained the enantioselectivity and diastereoselectivity. The overall mechanism consists of the coordination of the aldehyde, carbon–carbon bond formation, the rate-determining proton transfer from water to aldehyde, and dissociation of TMS group. The calculated full catalytic cycle is thus consistent with the experiments.
Access to paper
- Iron- and Bismuth-Catalyzed Asymmetric Mukaiyama Aldol Reactions in Aqueous Media
- T. Kitanosono, T. Ollevier, S. Kobayashi
- Chem. Asian J. , 8, 2873-2885 (2013). DOI: 10.1002/asia.201301149
- The Mechanism of Iron(II)-Catalyzed Asymmetric Mukaiyama Aldol Reaction in Aqueous Media: Density Functional Theory and Artificial Force-Induced Reaction Study
- W. M. C. Sameera, M. Hatanaka, T. Kitanosono, S. Kobayashi, K. Morokuma
- J. Am. Chem. Soc. , 137, 11085-11094 (2015). DOI: 10.1021/jacs.5b05835
究極のアルドール反応開発に向けて
Comment
The goal toward definitive catalytic asymmetric Mukaiyama aldol reaction is just around the corner. The intimate dependence of the catalyst structure on the substrate in highly selective Mukaiyama aldol reactions, a great problem for chemists, is nearly surmounted. The catalytic asymmetric Mukaiyama aldol reaction has been used even in pharmaceutical production. A Ti-BINOL complex was responsible under anhydrous conditions for the reaction of the ketene silyl acetal with the aldehyde to produce pitavastatin calcium (Livalo®), a cholesterol-lowering drug that belongs to one of the most potent statins on the market. Taking a wide range of application into account, there is room for improvement in the use of organic solvents, the reaction temperature, and the application to the reaction of ketene silyl acetals. These advances will have an immense impact on organic chemistry, providing an expedient, environmentally benign, and highly efficient pathway to accessing optically active aldols. We are now making serious efforts to establish a definitive varient of catalytic asymmetric aldol reactions.
Access to paper
- Iron- and Bismuth-Catalyzed Asymmetric Mukaiyama Aldol Reactions in Aqueous Media
- T. Kitanosono, T. Ollevier, S. Kobayashi
- Chem. Asian J. , 8, 2873-2885 (2013). DOI: 10.1002/asia.201301149
- The Mechanism of Iron(II)-Catalyzed Asymmetric Mukaiyama Aldol Reaction in Aqueous Media: Density Functional Theory and Artificial Force-Induced Reaction Study
- W. M. C. Sameera, M. Hatanaka, T. Kitanosono, S. Kobayashi, K. Morokuma
- J. Am. Chem. Soc. , 137, 11085-11094 (2015). DOI: 10.1021/jacs.5b05835