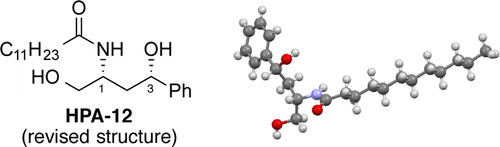
Revised Stereochemistry of Ceramide-Trafficking Inhibitor HPA-12 by X-ray Crystallography Analysis
In response to Berkeš’s report revising the stereochemistry of HPA-12, an important ceramide-trafficking inhibitor that was discovered and synthesized and its stereochemistry determined in 2001, the synthesis and the stereochemistry were reinvestigated. A large-scale synthetic method for HPA-12 based on a Zn-catalyzed asymmetric Mannich-type reaction in water was developed. Single crystals of HPA-12 for X-ray crystallographic analysis were obtained from ethyl propionate/n-hexane, and the stereochemistry was definitely determined to be 1R,3S, consistent with Berkeš’s revised structure.
Access to papers
- Revised Stereochemistry of Ceramide-Trafficking Inhibitor HPA-12 by X-ray Crystallography Analysis,
- M. Ueno, Y.-Y. Huang, A. Yamano, S. Kobayashi,
- Org. Lett., 15,2869-2871(2013). DOI: 10.1021/ol401101u