Development of catalytic asymmetric reactions using alkaline earth metal complexes
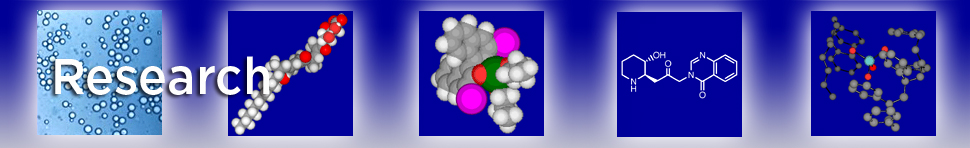
Alkaline earth metals are ubiquitous metals on the earth; however, their use as catalysts in organic synthesis has not been explored well. Our group has developed highly efficient catalysts for stereoselective carbon-carbon bond forming reactions by using alkaline earth metals, barium, calcium, and strontium.
Review:
- Chiral Ca-, Sr-, and Ba-Catalyzed Asymmetric Direct-Type Aldol, Michael, Mannich, and Related Reactions,
- T. Tsubogo, Y. Yamashita, S. Kobayashi,
- Top. Organomet. Chem., 45, 243-270 (2013). DOI: 10.1007/978-3-642-36270-5_7
Topics
- Chiral Ca-, Sr-, and Ba-Catalyzed Asymmetric Direct-Type Aldol, Michael, Mannich, and Related Reactions..
- Chiral Calcium Iodide for Asymmetric Mannich-type Reactions of Malonates with Imines Providing β-Aminocarbonyl Compounds
- Synthesis of Glutamic Acid and Highly Functionalized Pyrrolidine Derivatives by Utilizing Tunable Calcium Catalysts for Chemoselective Asymmetric 1,4-Addition and [3+2] Cycloaddition Reactions.
- Calcium Chloride (CaCl2) as Catalyst for Asymmetric Organic Reactions.
- Calcium-Catalyzed Bis-Hydrothiolation of Unactivated Alkynes Providing Dithioacetals.
Chiral Ca-, Sr-, and Ba-Catalyzed Asymmetric Direct-Type Aldol, Michael, Mannich, and Related Reactions.

Comment
Recent progress in asymmetric direct-type aldol, Michael, Mannich, and related reactions using chiral Ca, Sr, and Ba catalysts was summarized. Ca, Sr, and Ba are very attractive, because they are abundant and ubiquitous elements found in nature, and form relatively safe and environmentally benign compounds compared with heavy transition metals. However, their use as catalysts in asymmetric synthesis has been limited compared with that of transition metal catalysts. Their strong Brønsted basicity and mild Lewis acidity are promising and attractive characteristics, and can influence their catalytic activity as well as their chiral modification capability in a positive manner. It was revealed that several catalytic asymmetric carbon–carbon bond-forming and related reactions proceeded smoothly in high enantioselectivites using the chiral Ca, Sr, and Ba catalysts.
Access to paper
- Chiral Ca-, Sr-, and Ba-Catalyzed Asymmetric Direct-Type Aldol, Michael, Mannich, and Related Reactions,
- T. Tsubogo, Y. Yamashita, S. Kobayashi,
- Top. Organomet. Chem., 45, 243-270 (2013). DOI: 10.1007/978-3-642-36270-5_7
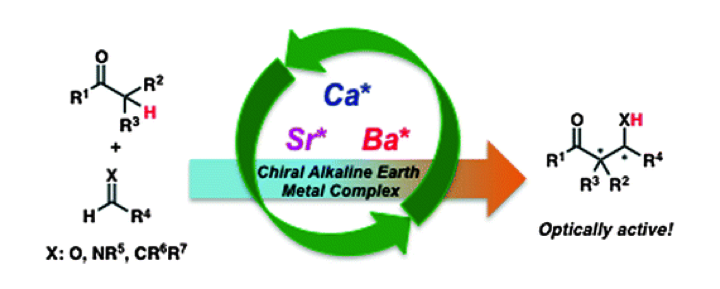
Chiral Calcium Iodide for Asymmetric Mannich-type Reactions of Malonates with Imines Providing β-Aminocarbonyl Compounds.
解説
We have developed a novel chiral calcium iodide catalyst from CaI2 and a Pybox that is stable under moisture and oxygen. This catalyst was applied to catalytic asymmetric Mannich-type reactions of malonates with both N-Boc-protected aromatic and aliphatic imines, and resulted in moderate to high yields with high enantioselectivities. To the best of our knowledge, this is the first example of highly enantioselective metal-catalyzed asymmetric Mannich-type reactions of malonates with N-Boc-protected aliphatic imines. The Mannich adduct was successfully converted into the α-hydroxy β-amino acid derivative.
Access to paper
- Chiral Calcium Iodide for Asymmetric Mannich-type Reactions of Malonates with Imines Providing β-Aminocarbonyl Compounds,
- T. Tsubogo, S. Shimizu, S. Kobayashi,
- Chem. Asian J., 8, 872-876 (2013). DOI: 10.1002/asia.201300102
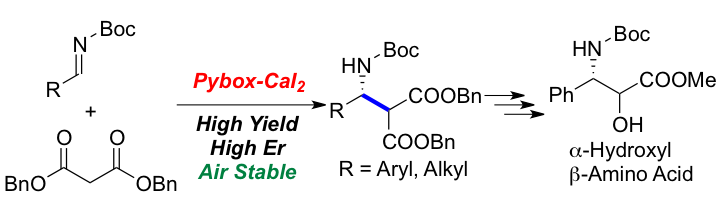
Synthesis of Glutamic Acid and Highly Functionalized Pyrrolidine Derivatives by Utilizing Tunable Calcium Catalysts for Chemoselective Asymmetric 1,4-Addition and [3+2] Cycloaddition Reactions.
Comment
We disclose asymmetric 1,4-addition and [3+2] cycloaddition reactions using simple catalytic systems consisting of calcium chloride dihydrate, Box-type ligand and tetramethylguanidine. Depending on the structure of both glycine Schiff bases and α,β-unsaturated compounds, the corresponding Michael adducts or pyrrolidine derivatives were obtained in moderate to high yields with high enantioselectivities. Modification of the catalytic system by using more Lewis acidic calcium salts such as calcium triflate and neutral Pybox-type ligands allows a tuning of the chemoselectivity and leads to suppression of the [3+2] cycloadition reactions. This methodology has broadened a synthetic route to β-branched glutamic acid derivatives and established calcium salts as useful and attractive catalysts for asymmetric catalysis.
Access to paper
- Synthesis of Glutamic Acid and Highly Functionalized Pyrrolidine Derivatives by Utilizing Tunable Calcium Catalysts for Chemoselective Asymmetric 1,4-Addition and [3+2] Cycloaddition Reactions,
- M. Hut'ka, T. Tsubogo, S. Kobayashi,
- Adv. Synth. Catal., 355, 1561-1569 (2013). DOI: 10.1002/adsc.201300171
Calcium Chloride (CaCl2) as Catalyst for Asymmetric Organic Reactions.
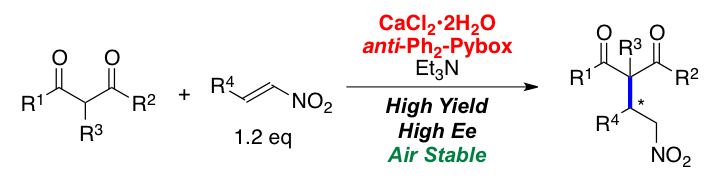
Comment
Pybox–CaCl2 was found to be an efficient chiral catalyst for asymmetric 1,4-addition reactions of 1,3-dicarbonyl compounds with nitroalkenes, affording γ-nitro carbonyl compounds in high yields with high enantioselectivities. The reactions proceeded smoothly even in air, and were successfully applied to a continuous flow system.
Access to paper
- Calcium Chloride (CaCl2) as Catalyst for Asymmetric Organic Reactions,
- T. Tsubogo, Y. Yamashita, S. Kobayashi,
- Topics in Catalysis, 57, 935-939 (2014). DOI: 10.1007/s11244-014-0254-z
Calcium-Catalyzed Bis-Hydrothiolation of Unactivated Alkynes Providing Dithioacetals.
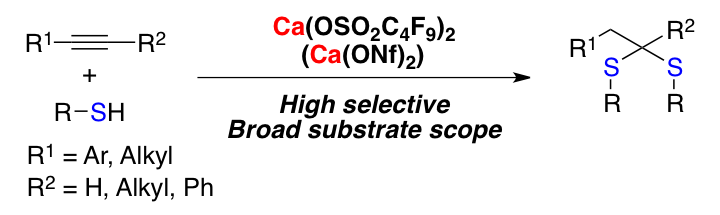
Comment
Bis-hydrothiolation of alkynes providing anti-Markovnikov dithioacetals is reported. Lewis-acidic Ca(OSO2C4F9)2 (Ca(ONf)2) was synthesized for the first time and was shown to be an excellent catalyst for the transformation. The reaction is highly selective and has a wide substrate scope. It was revealed that vinyl sulfides were intermediates for this transformation and that Ca(ONf)2 efficiently catalyzed the unprecedented reactions of unactivated vinyl sulfides with thiols to afford the dithioacetals in good to high yields.
Access to paper
- Calcium-Catalyzed Bis-Hydrothiolation of Unactivated Alkynes Providing Dithioacetals,
- M. Hut’ka, T. Tsubogo, S. Kobayashi,
- Organometallics, 33, 5626-5629 (2014). DOI: 10.1021/om500442u