Publication
- Article : 2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002
2024
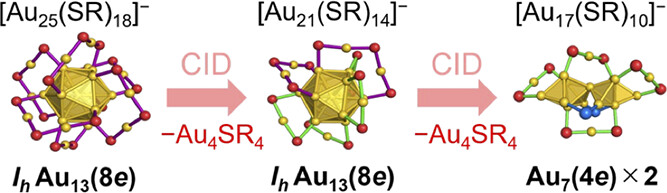
Gas-Phase Structures of [Au21(SR)14]– and [Au17(SR)10]– with Eight Electrons: Can They Support an Icosahedral Au13 Core?
Yuki Fujiwara, Shun Ito, Kiichirou Koyasu, and Tatsuya Tsukuda*
J. Phys. Chem. A (2024), in press.
Cyclic ion mobility of doped [MAu24L18]2– superatoms and their fragments (M = Ni, Pd and Pt; L = alkynyl)
Frank Hennrich, Shun Ito, Patrick Weis, Marco Neumaier, Shinjiro Takano, Tatsuya Tsukuda, and Manfred M. Kappes*
Phys. Chem. Chem. Phys., 26, 8408-8418 (2024).
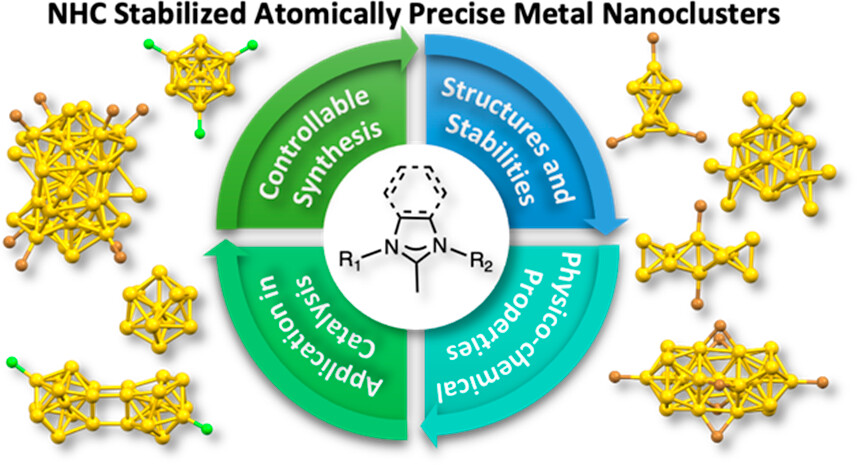
N-Heterocyclic Carbene-Stabilized Atomically Precise Metal Nanoclusters
Emily L. Albright, Tetyana I. Levchenko, Viveka K. Kulkarni, Angus I. Sullivan, Joseph F. DeJesus, Sami Malola, Shinjiro Takano, Masakazu Nambo*, Kevin Stamplecoskie*, Hannu Häkkinen*, Tatsuya Tsukuda*, and Cathleen M. Crudden*
J. Am. Chem. Soc., 146, 5759-5780 (2024).
Invited Perspective
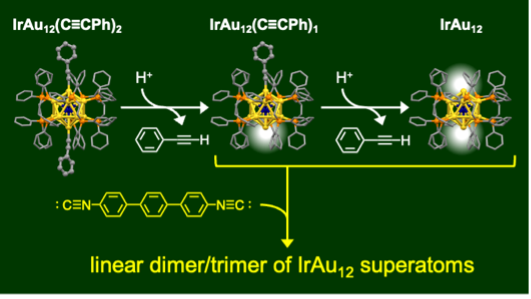
Diphosphine-Protected IrAu12 Superatom with Open Site(s): Synthesis and Programmed Stepwise Assembly
Yuto Fukumoto†, Tsubasa Omoda†, Haru Hirai, Shinjiro Takano, Koji Harano, and Tatsuya Tsukuda* (†: Equal contribution)
Angew. Chem., Int. Ed., e202402025 (2024).
Open Access
Featured in ChemistryViews
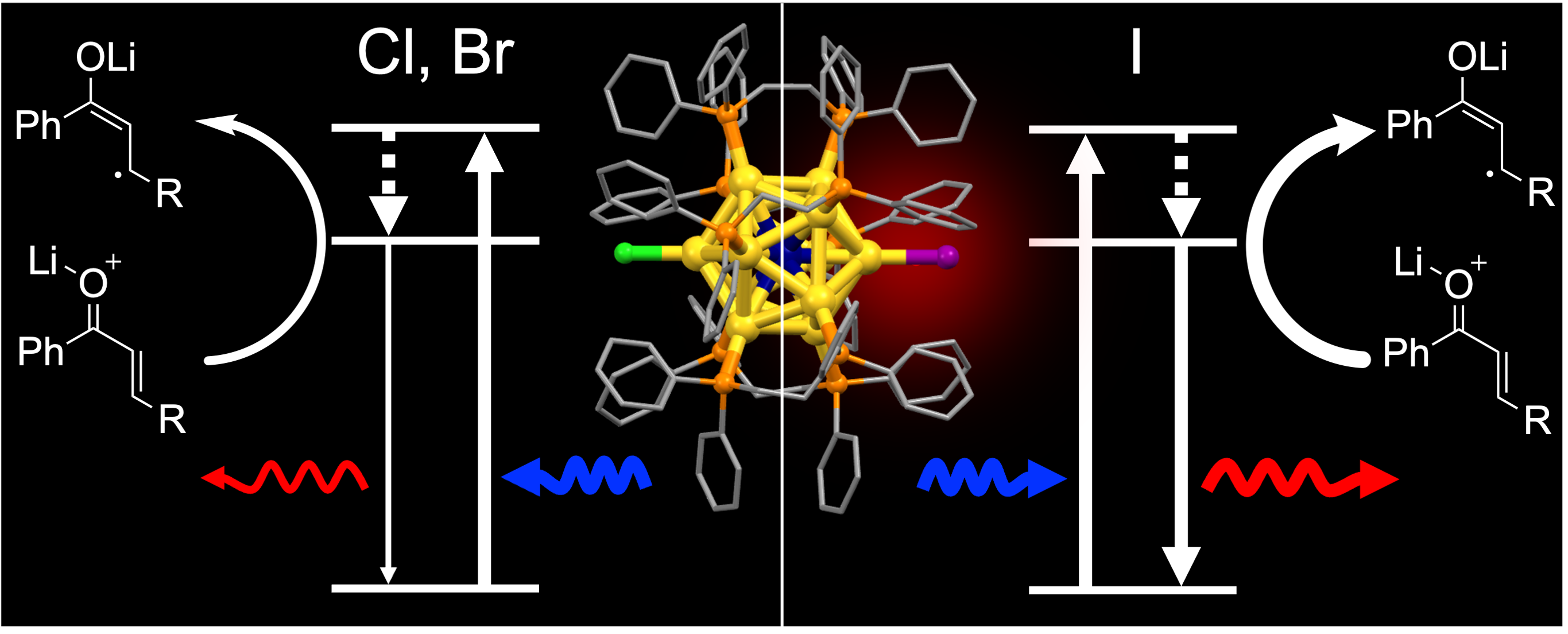
Introducing Iodide Ligands on IrAu12 Cluster Enhances Phosphorescence Efficiency and Photoredox Activity
Haru Hirai, Shinjiro Takano, Shinya Masuda, and Tatsuya Tsukuda*
ChemElectroChem, 11, e202300669 (2024).
Flavio Maran Festschrift, Open Access
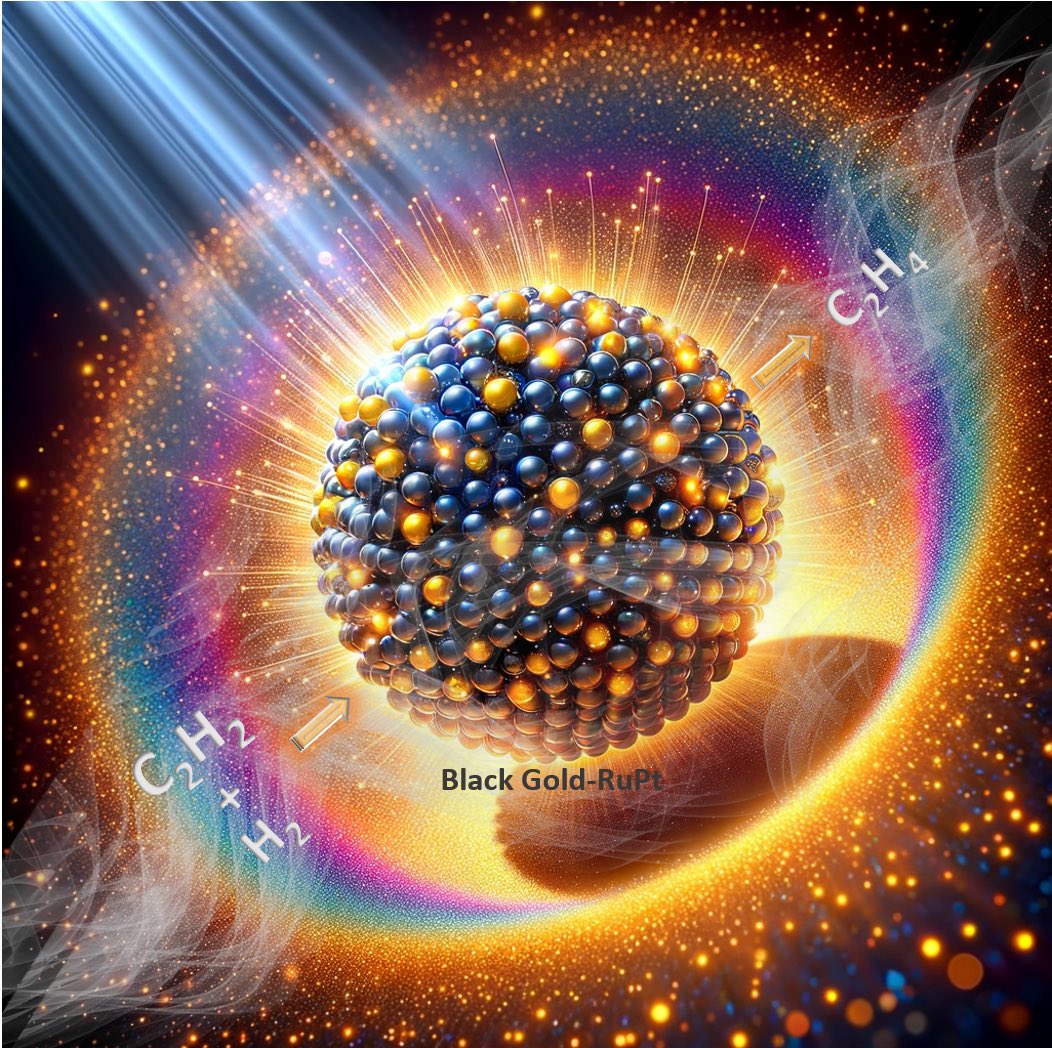
Pt-doped Ru nanoparticles loaded on 'black gold' plasmonic nanoreactors as air stable reduction catalysts
Gunjan Sharma, Rishi Verma, Shinya Masuda, Khaled Mohamed Badawy, Nirpendra Singh, Tatsuya Tsukuda*, and Vivek Polshettiwar*
Nature Communications, 15, 713 (2024).
Open Access
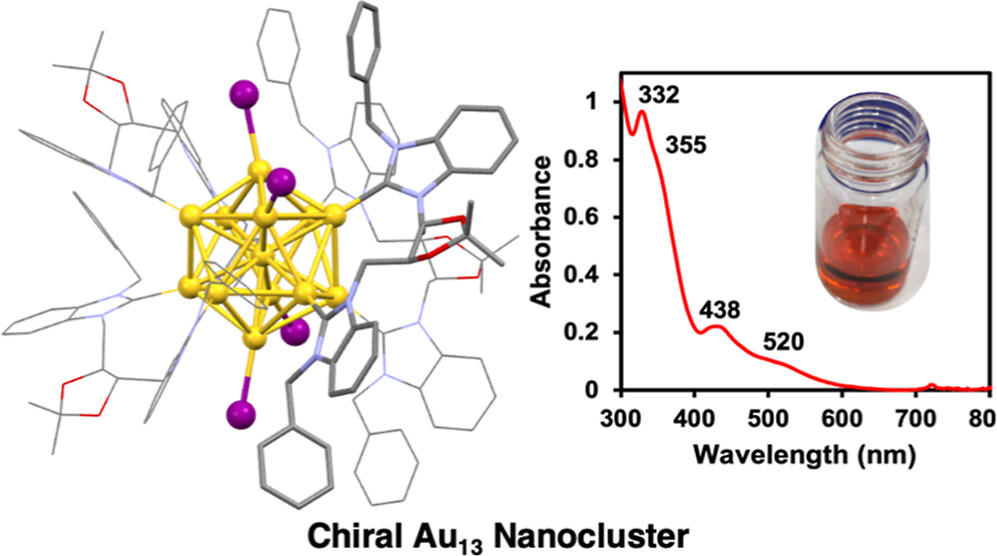
Enantiopure Chiral Au13 Nanoclusters Stabilized by Ditopic N-Heterocyclic Carbenes: Synthesis, Characterization, and Electrocatalytic Reduction of CO2
Emily L. Albright, Sami Malola, Samuel I. Jacob, Hong Yi, Shinjiro Takano, Koichi Mimura, Tatsuya Tsukuda*, Hannu Häkkinen*, Masakazu Nambo*, and Cathleen M. Crudden*
Chem. Matter., 36, 1279-1289 (2024).
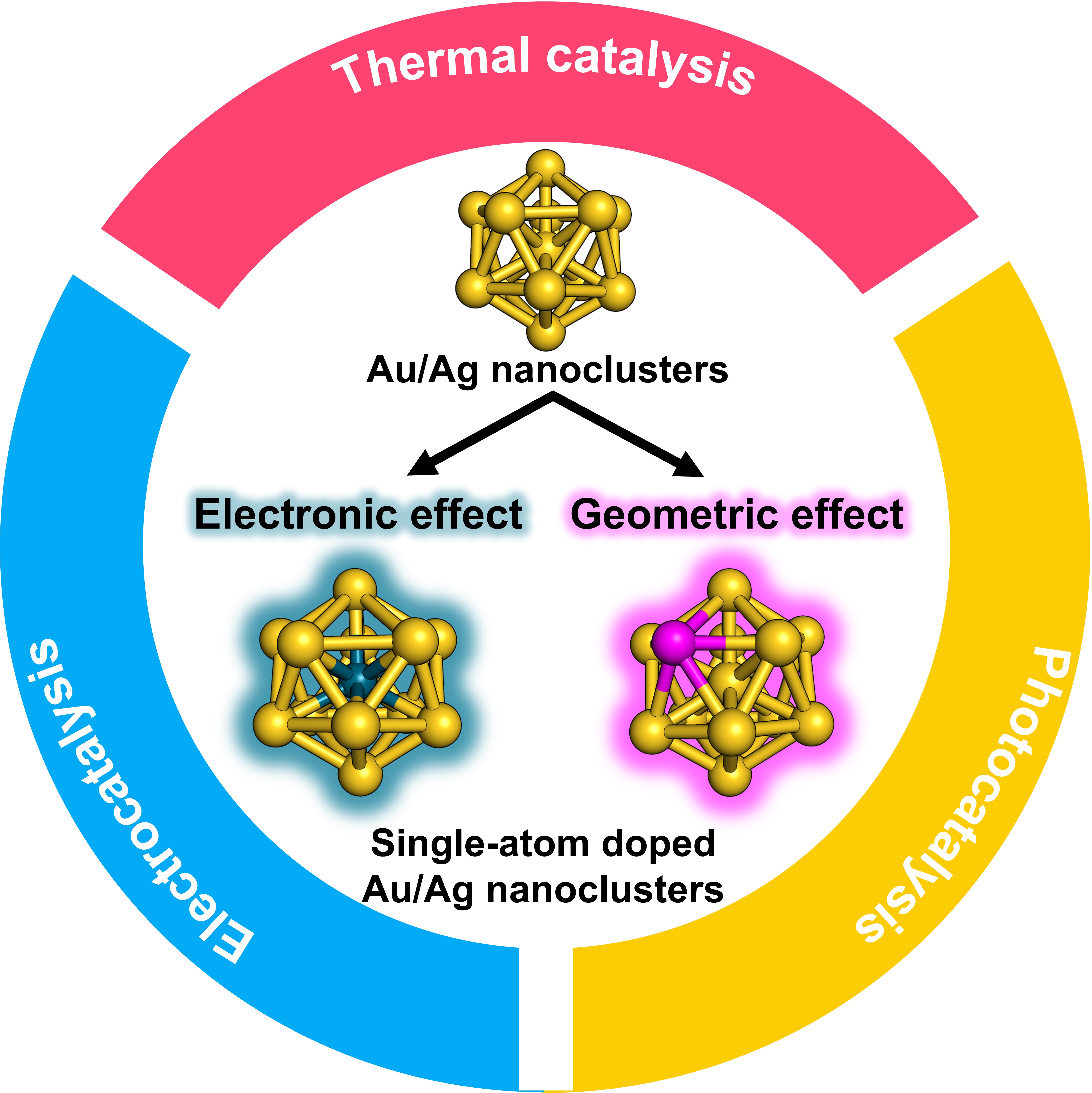
Atomically precise Au and Ag nanoclusters doped with single atom as model alloy catalysts
Shinya Masuda, Kosuke Sakamoto, and Tatsuya Tsukuda*
Nanoscale, 16, 4514-4528 (2024).
Invited Minireview, Open Access
Selected as Front Cover

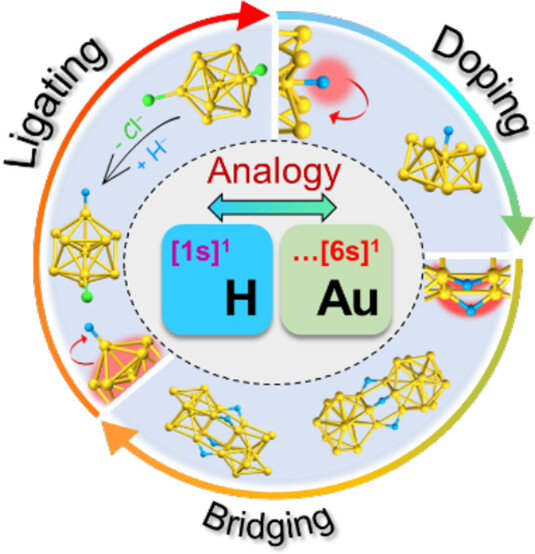
Bonding and Electronic Interactions of Hydrogen with Gold Superatoms
Subarna Maity, Shinjiro Takano, Shinya Masuda, and Tatsuya Tsukuda*
J. Phys. Chem. C, 128, 19-30 (2024).
Invited Perspective
Selected as Supplementary Cover Image