Theoretical Studies on the Addition of Polymetallic Lithium
Organocuprate Clusters to Acetylene. Cooperative Effects of Metals in
a Trap-and-Bite Reaction Pathway
Eiichi Nakamura*, Seiji Mori, Masaharu Nakamura, and Keiji
Morokuma**
J. Am. Chem. Soc., 119, 4887-4899 (1997)
This article contains 3D-pictures. In order to see these moving
pictures, you need to have a Chemscape
Chimie plug-in in your Netscape Navigator plug- in folder.
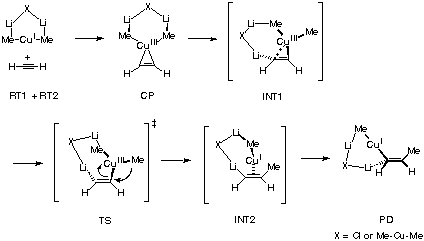
Ab initio and density functional theoretical investigations into the
nature of the reaction of acetylene with non-cluster organocuprate
reagents, MeCu, Me2Cu-, and lithium organocuprate cluster reagents,
Me2CuLi, Me2CuLi„LiCl, and (Me2CuLi)2 were carried out, and the
function of the mixed metal cluster was probed. In the cluster
reaction of Me2CuLi„LiCl, the lithium atom in the cluster
stabilizes the developing negative charge on the acetylene
moiety, and assists the electron flow from the copper atom.
Reductive elimination of the transient Cu(III) species
initially gives a 1-propenyl-lithium-like structure intermediate
(non-stationary point), which then undergo intramolecular
transmetalation to give the final product, 1-propenylcopper.
Essentially the same mechanism operates also with Me2CuLi and
(Me2CuLi)2, indicating that the Li-Me-Cu-Me moiety incorporated in
the mixed organocuprate cluster is essential for the reaction.
Return
to 3D Structures of Organometallic reaction Pathway