Complexation of Lewis Acid with Trialkylcopper(III). On the
Origin of BF3-Acceleration of Cuprate Conjugate
Addition
Eiichi Nakamura* and Masahiro Yamanaka and Seiji Mori
J. Am. Chem. Soc. 122, 1826-1827 (2000)
This article contains 3D-pictures. In order to see these moving
pictures, you need to have a Chemscape
Chimie plug-in in your Netscape Navigator plug- in folder.
It has been widely appreciated and extensively exploited in synthesis that BF3
accelerates conjugate addition of an organocopper reagent. In the light of
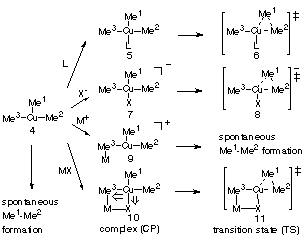
the involvement of a b-cuprio(III) enolate intermediate
in the rate limiting step of the conjugate addition, the effects of Lewis acids
(LiCl dimer and BF3) on the kinetic and thermodynamic behavior of Me3Cu(III)
were examined with the density functional method B3LYP and coupled cluster method
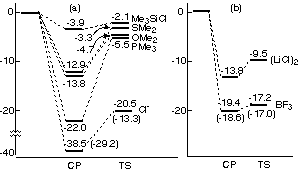
CCSD(T). The studies showed that a Lewis acid strongly stabilizes Me3Cu(III)
through formation of a four-centered complex, while keeping the activation barrier
of reductive elimination very low (a few kcal/mol). This property of a Lewis
acid stands in contrast to that of a Lewis base such as Me2O, Me2S, Me3P, and
chloride anion, all of which stabilize Me3Cu(III) while greatly increasing the
energy barrier of reductive elimination (to ca. 10 to 20 kcal/mol). The present
findings suggest that a BF3 controls the rate and the stereochemistry
of the conjugate addition through participation in the reductive elimination of
the copper(III) intermediate.
|
|
Å@
|
Return
to 3D Structures of Organometallic reaction Pathway